What is Mounjaro?
- Mounjaro is an injectable prescription medicine that is used along with diet and exercise to improve blood sugar (glucose) in adults with type 2 diabetes mellitus.
- It is not known if Mounjaro can be used in people who have had pancreatitis.
- Mounjaro is not for use in people with type 1 diabetes.
- It is not known if Mounjaro is safe and effective for use in children under 18 years of age.
What is the most important information I should know about Mounjaro?
Mounjaro may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rats, Mounjaro and medicines that work like Mounjaro caused thyroid tumors, including thyroid cancer. It is not known if Mounjaro will cause thyroid tumors, or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
- Do not use Mounjaro if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC), or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Who should not use Mounjaro?
Do not use Mounjaro if:
- you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- you have had a serious allergic reaction to tirzepatide or any of the ingredients in Mounjaro. See below for a complete list of ingredients in Mounjaro.
What should I tell my healthcare provider before using Mounjaro?
Before using Mounjaro, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had problems with your pancreas or kidneys.
- have severe problems with your stomach, such as slowed emptying of your stomach (gastroparesis) or problems with digesting food.
- have a history of diabetic retinopathy.
- are pregnant or plan to become pregnant. It is not known if Mounjaro will harm your unborn baby. Tell your healthcare provider if you become pregnant while using Mounjaro.
- Birth control pills by mouth may not work as well while using Mounjaro. If you take birth control pills by mouth, your healthcare provider may recommend another type of birth control for 4 weeks after you start Mounjaro and for 4 weeks after each increase in your dose of Mounjaro. Talk to your healthcare provider about birth control methods that may be right for you while using Mounjaro.
- are breastfeeding or plan to breastfeed. It is not known if Mounjaro passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while using Mounjaro.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Mounjaro may affect the way some medicines work, and some medicines may affect the way Mounjaro works.
Before using Mounjaro, tell your healthcare provider if you are taking other medicines to treat diabetes including insulin or sulfonylureas which could increase your risk of low blood sugar. Talk to your healthcare provider about low blood sugar and how to manage it.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Mounjaro?
- Read the Instructions for Use that comes with Mounjaro.
- Use Mounjaro exactly as your healthcare provider tells you to.
- Mounjaro is injected under the skin (subcutaneously) of your stomach (abdomen), thigh, or upper arm.
- Use Mounjaro 1 time each week, at any time of the day.
- You may change the day of the week you use Mounjaro as long as the time between the 2 doses is at least 3 days (72 hours).
- If you miss a dose of Mounjaro, take the missed dose as soon as possible within 4 days (96 hours) after the missed dose. If more than 4 days have passed, skip the missed dose and take your next dose on the regularly scheduled day. Do not take 2 doses of Mounjaro within 3 days of each other.
- Mounjaro may be taken with or without food.
- Do not mix insulin and Mounjaro together in the same injection.
- You may give an injection of Mounjaro and insulin in the same body area (such as your stomach area), but not right next to each other.
- Change (rotate) your injection site with each weekly injection. Do not use the same site for each injection.
- If you take too much Mounjaro, call your healthcare provider.
What are the possible side effects of Mounjaro?
Mounjaro may cause serious side effects, including:
- See “What is the most important information I should know about Mounjaro?”
- inflammation of your pancreas (pancreatitis). Stop using Mounjaro and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
- low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Mounjaro with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include:
- dizziness or light-headedness
- blurred vision
- anxiety, irritability, or mood changes
- sweating
- slurred speech
- hunger
- confusion or drowsiness
- shakiness
- weakness
- headache
- fast heartbeat
- feeling jittery
- serious allergic reactions. Stop using Mounjaro and get medical help right away if you have any symptoms of a serious allergic reaction including:
- swelling of your face, lips, tongue or throat
- problems breathing or swallowing
- severe rash or itching
- fainting or feeling dizzy
- very rapid heartbeat
- kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration.
- severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Mounjaro. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
- changes in vision. Tell your healthcare provider if you have changes in vision during treatment with Mounjaro.
- gallbladder problems. Gallbladder problems have happened in some people who use Mounjaro. Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include:
- pain in your upper stomach (abdomen)
- fever
- yellowing of skin or eyes (jaundice)
- clay-colored stools
The most common side effects of Mounjaro include:
- nausea
- diarrhea
- decreased appetite
- vomiting
- constipation
- indigestion
- stomach (abdominal) pain
Talk to your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Mounjaro. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Mounjaro
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Mounjaro for a condition for which it was not prescribed. Do not give Mounjaro to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Mounjaro that is written for health professionals.
How should I store Mounjaro?
- Store Mounjaro in the refrigerator between 36⁰F to 46⁰F (2⁰C to 8⁰C). Store Mounjaro in the original carton until use to protect it from light.
- If needed, each single-dose pen can be stored at room temperature up to 86⁰F (30⁰C) for up to 21 days.
- Do not freeze Mounjaro. Do not use Mounjaro if frozen.
Keep Mounjaro and all medicines out of the reach of children.
What are the ingredients in Mounjaro?
Active ingredient: tirzepatide
Inactive ingredients: sodium chloride, sodium phosphate dibasic heptahydrate, and water for injection. Hydrochloric acid solution and/or sodium hydroxide solution may have been added to adjust the pH.
For more information, go to www.MOUNJARO.com or call 1-800-545-5979.
Instructions for use for Mounjaro
Mounjaro (mown-JAHR-OH)
(tirzepatide)
injection, for subcutaneous use
Important information you need to know before injecting Mounjaro
Read this Instructions for Use and the Medication Guide before using your Mounjaro Pen and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Talk to your healthcare provider about how to inject Mounjaro the right way.
- Mounjaro is a single-dose prefilled pen.
- Mounjaro is used 1 time each week.
- Inject under the skin (subcutaneously) only.
- You or another person can inject into your stomach (abdomen) or thigh.
- Another person can inject into the back of your upper arm.
Storage and handling
- Store your Pen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store your Pen at room temperature up to 86°F (30°C) for up to 21 days.
- Do not freeze your Pen. If the Pen has been frozen, throw the Pen away and use a new Pen.
- Store your Pen in the original carton to protect your Pen from light.
- The Pen has glass parts. Handle it carefully. If you drop the Pen on a hard surface, do not use it. Use a new Pen for your injection.
- Keep your Mounjaro Pen and all medicines out of the reach of children.
Guide to parts
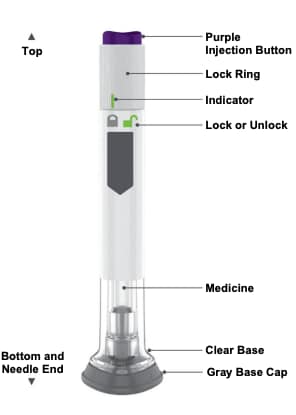
Preparing to inject Mounjaro
- Remove the Pen from the refrigerator. Leave the gray base cap on until you are ready to inject.
- Check the Pen label to make sure you have the right medicine and dose, and that it has not expired.
- Inspect the Pen to make sure that it is not damaged.

Make sure the medicine:
- is not frozen
- is not cloudy
- is colorless to slightly yellow
- does not have particles
Wash your hands.
Step 1. Choose your injection site
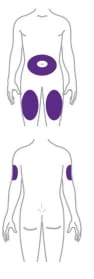
- Your healthcare provider can help you choose the injection site that is best for you.
- You or another person can inject the medicine in your stomach (abdomen) or thigh.
- Another person should give you the injection in the back of your upper arm.
- Change (rotate) your injection site each week.
- You may use the same area of your body but be sure to choose a different injection site in that area.
Step 2. Pull off the gray base cap
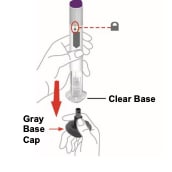
- Make sure the Pen is locked.
- Do not unlock the Pen until you place the clear base on your skin and are ready to inject.
- Pull the gray base cap straight off and throw it away in your household trash.
- Do not put the gray base cap back on – this could damage the needle.
- Do not touch the needle.
Step 3. Place clear base on skin, then unlock
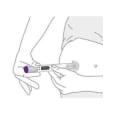
- Place the clear base flat against your skin at the injection site.
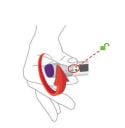
- Unlock by turning the lock ring.
Step 4. Press and hold up to 10 seconds
- Press and hold the purple injection button for up to 10 seconds. Listen for:
- First click = injection started
- Second click = injection completed You will know your injection is complete when the gray plunger is visible.
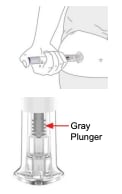
- You will know your injection is complete when the gray plunger is visible
After your injection, place the used Pen in a sharps container. See Disposing of your used Pen.
Disposing of your used Pen
- Put your used Pen in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) Pens in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.
Commonly asked questions
What if I see air bubbles in my Pen?
Air bubbles are normal.
What if my Pen is not at room temperature?
It is not necessary to warm the Pen to room temperature.
What if I unlock the Pen and press the purple injection button before pulling off the gray base cap?
Do not remove the gray base cap. Throw away the Pen and get a new Pen.
What if there is a drop of liquid on the tip of the needle when I remove the gray base cap?
A drop of liquid on the tip of the needle is normal. Do not touch the needle.
Do I need to hold the injection button down until the injection is complete?
This is not necessary, but it may help you keep the Pen steady against your skin.
I heard more than 2 clicks during my injection—2 loud clicks and 1 soft one. Did I get my complete injection?
Some people may hear a soft click right before the second loud click. That is the normal operation of the Pen. Do not remove the Pen from your skin until you hear the second loud click.
I am not sure if my Pen worked the right way.
Check to see if you have received your dose. Your dose was delivered the right way if the gray plunger is visible. Also, see Step 4 of the instructions.

If you do not see the gray plunger, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) for further instructions. Until then, store your Pen safely to avoid an accidental needle stick.
What if there is a drop of liquid or blood on my skin after my injection?
This is normal. Press a cotton ball or gauze over the injection site. Do not rub the injection site.
Other information
If you have vision problems, do not use your Pen without help from a person trained to use the Mounjaro Pen.
Where to learn more
- If you have questions or problems with your Mounjaro Pen, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) or call your healthcare provider.
- For more information about the Mounjaro Pen, visit our website at www.Mounjaro.com.
Instructions for use approved 05/2022




