What is naloxone hydrochloride?
Naloxone hydrochloride injection is indicated for use by military personnel and chemical incident responders for:
- Emergency treatment of patients 12 years of age and older where use of high-potency opioids such as fentanyl analogues as a chemical weapon is suspected.
- Temporary prophylaxis of respiratory and/or central nervous system depression in military personnel and chemical incident responders entering an area contaminated with high-potency opioids such as fentanyl analogues.
What is the most important information I should know about naloxone hydrochloride?
Important:
- Administer naloxone hydrochloride injection as soon as possible after known or suspected opioid exposure.
- If an individual is unresponsive and opioid exposure is suspected, administer naloxone hydrochloride injection as quickly as possible because an opioid emergency can cause severe injury or death.
- Each naloxone hydrochloride injection auto-injector can only be used 1 time.
You do not need to assemble your auto-injector. It comes already assembled for use.
How should I use naloxone hydrochloride?
- Read the Instructions for Use that comes with naloxone hydrochloride injection before using it.
How should I store naloxone hydrochloride
- Store naloxone hydrochloride injection auto-injector at room temperature between 59°F to 77°F (15°C to 25°C). Storage temperature excursions permitted between 39°F to 104°F (4°C to 40°C).
- Protect from heat and do not freeze.
- Keep the naloxone hydrochloride injection auto-injector in its outer case until ready to use
- Occasionally check the viewing window of the Naloxone hydrochloride injection auto-injector. If the medicine is cloudy, contains particles, or if the glass container is damaged, replace the auto-injector with a new one. Naloxone hydrochloride injection auto-injector has an expiration date. Replace it before the expiration date.
Keep Naloxone hydrochloride injection and all medicines out of the reach of children.
What are the ingredients in naloxone hydrochloride?
Active ingredients: naloxone hydrochloride
Inactive ingredients: sodium chloride, hydrochloric acid to adjust pH, water for injection.
Instructions for use for naloxone hydrochloride
Naloxone hydrochloride injection (Nuh-laak-sown hai-drow-klaw-ride) for intramuscular or subcutaneous use
Read the Instructions for Use that comes with naloxone hydrochloride injection before using it.
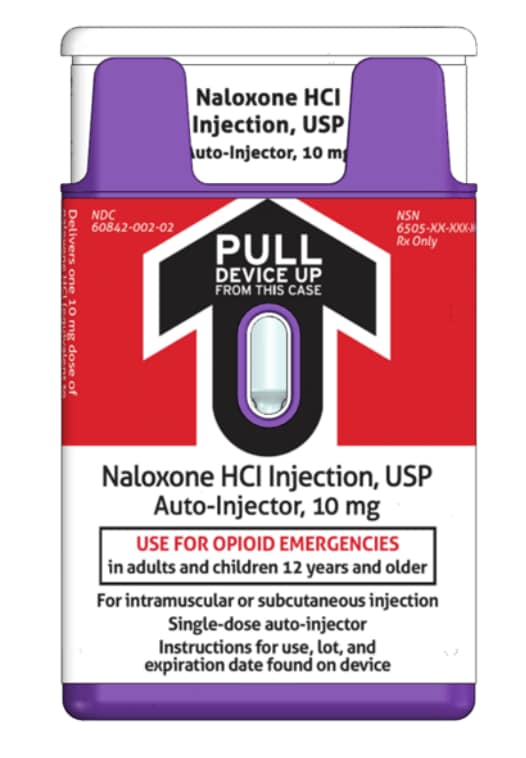
Naloxone hydrochloride injection comes in a single-dose, pre-filled auto-injector.
Figure A – Naloxone hydrochloride injection Kaleo auto-injector Parts
Naloxone hydrochloride injection auto-injector inside the Outer Case - Before Use
Naloxone hydrochloride injection auto-injector with Outer Case removed
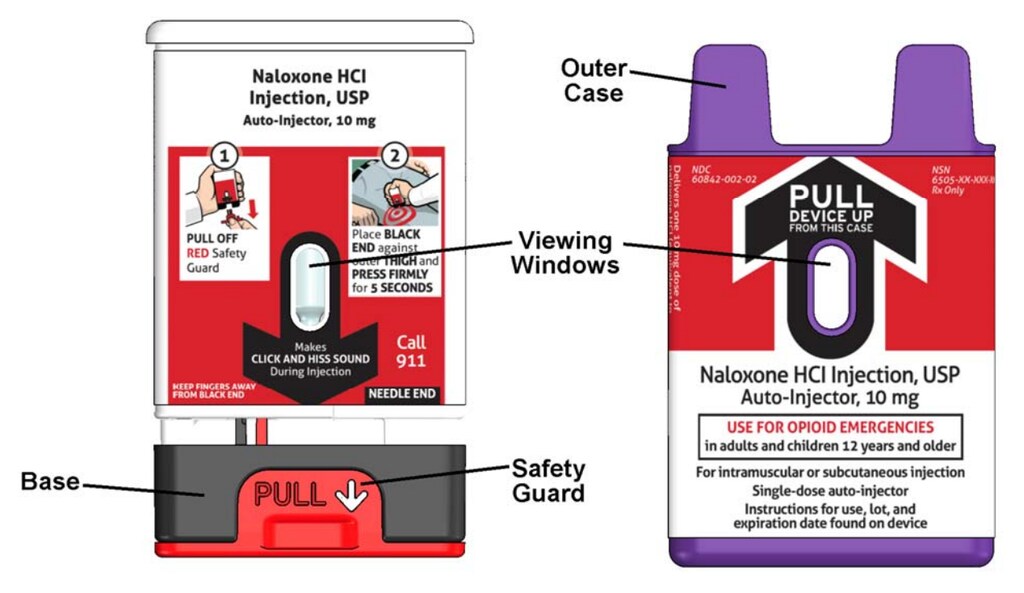
Important note: Before you use the naloxone hydrochloride injection auto-injector, look at the viewing window. Do not use the auto-injector if the medicine is cloudy or contains particles or if there is a red indicator in the viewing window. Use a new naloxone hydrochloride injection auto-injector.
How to use the auto-injector
Step 1.
Firmly pull the auto-injector from the outer case. See Figure B.
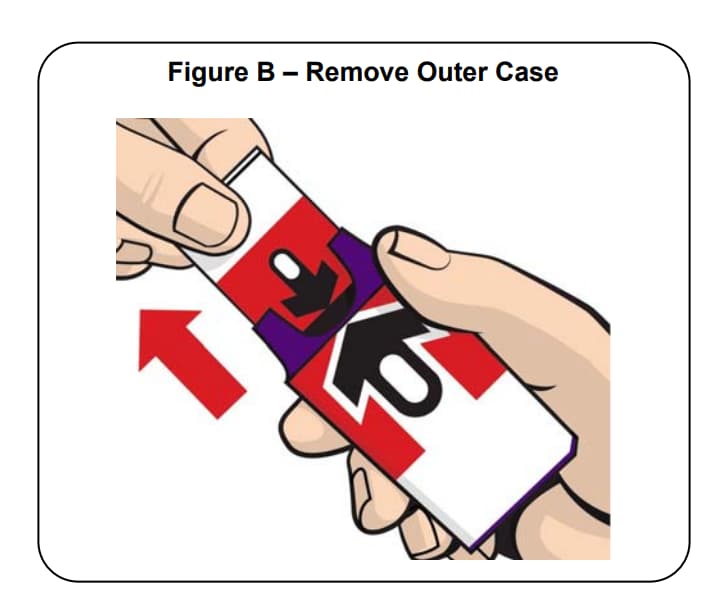
Do not go to Step 2 (Do not remove the Red safety guard) until you are ready to use the auto-injector. If. you are not ready to use the auto-injector, put it back in the outer case for later use.
If naloxone hydrochloride injection auto-injector is frozen, do not wait for it to thaw. Use another auto-injector if available. If another auto-injector is not available, get emergency medical help right away.
Naloxone hydrochloride injection auto-injector may still be used if it has been thawed after being previously frozen.
Step 2.
Pull off the Red safety guard. See Figure C.
To reduce the chance of an accidental injection, do not touch the Black base of the auto-injector, which is where the needle comes out.
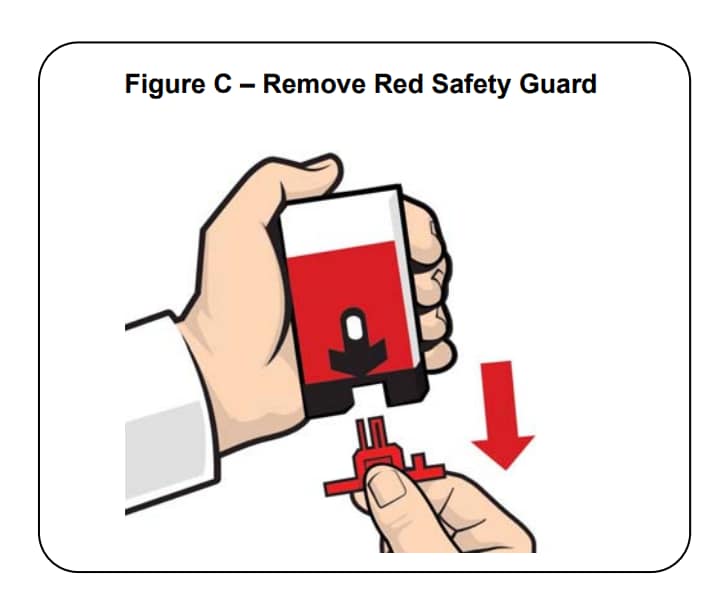
Note: The Red safety guard is made to fit tightly. Pull firmly to remove.
Do not replace the Red safety guard after it is removed.
Step 3.
Place the Black end of the auto-injector against the outer thigh, through clothing or personal protective equipment (including MOPP4 PPE), if needed. Make sure that the injection site is free of other materials such as equipment or other obstructions, except for clothing. Naloxone hydrochloride injection auto-injector may be self-administered or administered by a buddy.
Press firmly until you hear a click and hiss sound and then hold in place for 5 seconds. See Figure D.
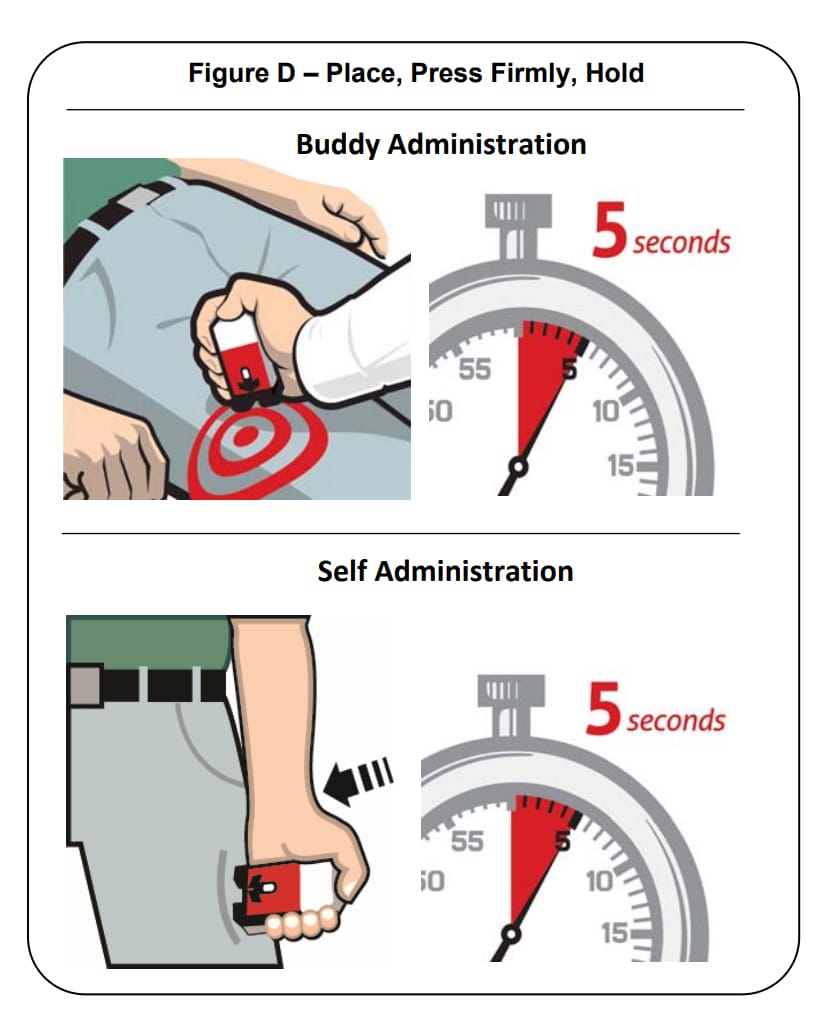
Note: The auto-injector makes a distinct sound (click and hiss) when it is pressed against the thigh. The click and hiss indicates the start of the injection. This is normal and means that the auto-injector is working correctly. Keep the auto-injector firmly pressed against the thigh for 5 seconds after you hear the click and hiss sound. After the medicine is injected, the needle will retract back up into the auto-injector and is not visible after use.
Step 4.
After administering the first dose of Naloxone hydrochloride injection, get emergency medical help right away.
Rescue breathing or CPR (cardiopulmonary resuscitation) may be given while waiting for emergency medical help. If there is no response to naloxone hydrochloride injection consider if respiratory depression is caused by something other than opioid exposure. Watch the person closely. If symptoms return, additional naloxone may be administered.
You may need additional doses of naloxone hydrochloride injection if you are being exposed to high-potency opioids for an extended period of time. Naloxone hydrochloride injection auto-injector provides temporary protection against opioid exposure.
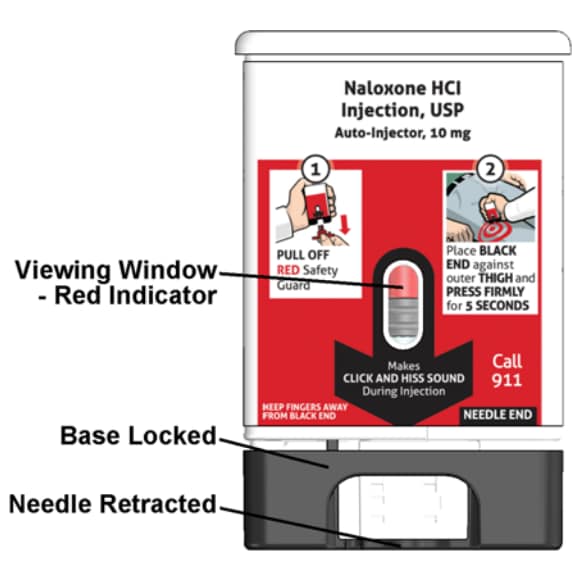
How to know that the auto-injector has been used.
See Figure E.
- The viewing window will no longer be clear. You will see a Red indicator.
- The Black base will lock into place.
Figure E - Naloxone hydrochloride injection auto-injector – After Use
What to do after the auto-injector has been used:
- Get emergency medical help right away.
- The auto-injector cannot be reused. Put the auto-injector back in the outer case after it has been used.
- Do not throw away the auto-injector in the trash. Do not recycle the auto-injector.
- The auto-injector must be properly disposed of in a sharps container.
There may be local or state laws about how to throw away used auto-injectors.
How should I store naloxone hydrochloride injection?
- Store naloxone hydrochloride injection auto-injector at room temperature between 59°F to 77°F (15°C to 25°C). Storage temperature excursions permitted between 39°F to 104°F (4°C to 40°C).
- Protect from heat and do not freeze.
- Keep the naloxone hydrochloride injection auto-injector in its outer case until ready to use
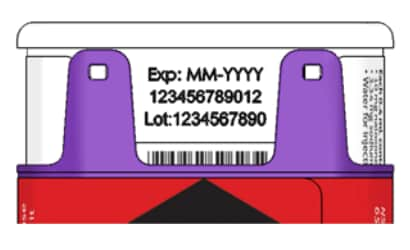
- Occasionally check the viewing window of the naloxone hydrochloride injection auto-injector. If the medicine is cloudy, contains particles, or if the glass container is damaged, replace the auto-injector with a new one. Naloxone hydrochloride injection auto-injector has an expiration date. See Figure F. Replace it before the expiration date.
Figure F – Expiration Date Located on Back of the naloxone hydrochloride injection auto-injector
Keep naloxone hydrochloride injection and all medicines out of the reach of children.
Instruction for use issued 02/2022.




