What is Prezista?
Prezista is a prescription HIV-1 (Human Immunodeficiency Virus-type 1) medicine used with ritonavir and other antiretroviral medicines to treat HIV-1 infection in adults and children 3 years of age and older. HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
Prezista should not be used in children under 3 years of age.
When used with other antiretroviral medicines to treat HIV-1 infection, Prezista may help:
- reduce the amount of HIV-1 in your blood. This is called "viral load".
- increase the number of CD4+ (T) cells in your blood that help fight off other infections.
Reducing the amount of HIV-1 and increasing the CD4+ (T) cells in your blood may improve your immune system. This may reduce your risk of death or getting infections that can happen when your immune system is weak (opportunistic infections).
Prezista does not cure HIV-1 infection or AIDS. You must keep taking HIV-1 medicines to control HIV-1 infection and decrease HIV-related illnesses.
Avoid doing things that can spread HIV-1 infection to others:
- Do not share or re-use needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
Ask your healthcare provider if you have any questions on how to prevent passing HIV to other people.
What is the most important information I should know about Prezista?
- Ask your healthcare provider or pharmacist about medicines that should not be taken with Prezista. For more information, see “Who should not take Prezista?" and “What should I tell my healthcare provider before taking Prezista?"
- Prezista may cause liver problems. Some people taking Prezista in combination with ritonavir have developed liver problems, which may be life-threatening. Your healthcare provider should do blood tests before and during your Prezista and ritonavir combination treatment. If you have chronic hepatitis B or C infection, your healthcare provider should check your blood tests more often because you have an increased chance of developing liver problems. Tell your healthcare provider if you have any of the below signs and symptoms of liver problems.
- dark (tea colored) urine
- yellowing of your skin or whites of your eyes
- pale colored stools (bowel movements)
- nausea
- vomiting
- pain or tenderness on your right side below your ribs
- loss of appetite
- tiredness
- Prezista may cause severe or life-threatening skin reactions or rash. Sometimes these skin reactions and skin rashes can become severe and require treatment in a hospital. Tell your healthcare provider right away if you develop a rash. Stop taking Prezista and ritonavir combination treatment and tell your healthcare provider right away if you have any skin changes with symptoms below:
- fever
- tiredness
- muscle or joint pain
- blisters or skin lesions
- mouth sores or ulcers
- red or inflamed eyes, like "pink eye" (conjunctivitis)
Rash occurred more often in people taking Prezista and raltegravir together than with either drug separately, but was generally mild.
See “What are the possible side effects of Prezista?" for more information about side effects.
Who should not take Prezista?
Do not take Prezista with any medicine that contains:
- alfuzosin
- cisapride
- colchicine, if you have liver or kidney problems
- dronedarone
- elbasvir and grazoprevir
- ergot-containing medicines:
- dihydroergotamine
- ergotamine tartrate
- methylergonovine
- ivabradine
- lomitapide
- lovastatin
- lurasidone
- midazolam, when taken by mouth
- naloxegol
- pimozide
- ranolazine
- rifampin
- sildenafil, when used for the treatment of pulmonary arterial hypertension (PAH)
- simvastatin
- St. John's wort (Hypericum perforatum)
- triazolam
Serious problems can happen if you or your child take any of these medicines with Prezista.
What should I tell my healthcare provider before taking Prezista?
Before taking Prezista, tell your healthcare provider if you:
- have liver problems, including hepatitis B or hepatitis C
- are allergic to sulfa medicines
- have high blood sugar (diabetes)
- have hemophilia
- have any other medical conditions
- are pregnant or plan to become pregnant. Tell your healthcare provider if you become pregnant while taking Prezista.
- Pregnancy Registry: There is a pregnancy registry for women who take antiretroviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take Prezista.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- It is not known if Prezista can pass into your breast milk.
- Talk to your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines interact with Prezista. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Prezista.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Prezista with other medicines.
How should I take Prezista?
- Take Prezista exactly as your healthcare provider tells you.
- You must take ritonavir at the same time as Prezista.
- Do not change your dose or stop treatment with Prezista without talking to your healthcare provider.
- Take Prezista and ritonavir with food.
- If you have difficulty swallowing Prezista tablets, Prezista oral suspension is also available. Your healthcare provider will help decide whether Prezista tablets or oral suspension is right for you.
- If your child is taking Prezista, your child's healthcare provider will decide the right dose based on your child's weight. Your child's healthcare provider will tell you how much Prezista (tablets or oral suspension) and how much ritonavir (capsules, tablets or solution) your child should take. Your child should take Prezista with ritonavir with food. If your child does not tolerate ritonavir oral solution, ask your child's healthcare provider for advice.
- Prezista oral suspension should be given with the supplied oral dosing syringe. Shake the suspension well before each use. See the instructions for use that come with Prezista oral suspension for information about the right way to prepare and take a dose.
- It is important that you do not miss or skip doses of Prezista during treatment.
- If you take too much Prezista, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Prezista?
Prezista may cause serious side effects, including:
- See “What is the most important information I should know about Prezista?"
- Diabetes and high blood sugar (hyperglycemia). Some people who take protease inhibitors including Prezista can get high blood sugar, develop diabetes, or your diabetes can get worse. Tell your healthcare provider if you notice an increase in thirst or urinate often while taking Prezista.
- Changes in body fat can happen in people who take HIV-1 medicines. The changes may include an increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the middle of your body (trunk). Loss of fat from the legs, arms, and face may also happen. The exact cause and long-term health effects of these conditions are not known.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after starting your HIV-1 medicine.
- Increased bleeding for hemophiliacs. Some people with hemophilia have increased bleeding with protease inhibitors including Prezista.
The most common side effects of Prezista include:
- diarrhea
- nausea
- rash
- headache
- stomach-area (abdominal) pain
- vomiting
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of Prezista.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
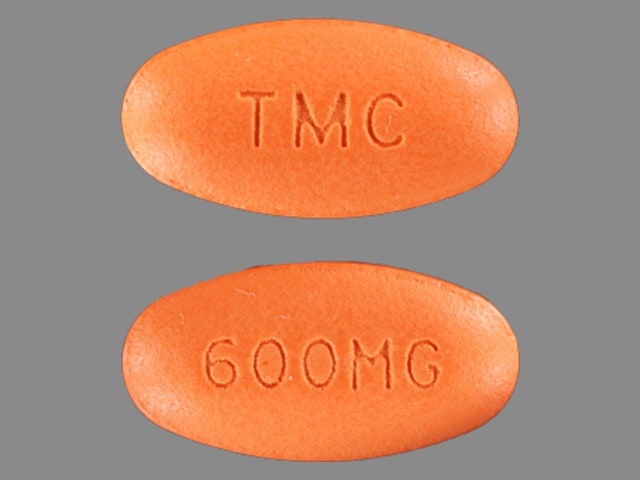
Prezista Images
General information about the safe and effective use of Prezista
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Prezista for a condition for which it was not prescribed. Do not give Prezista to other people even if they have the same condition you have. It may harm them.
This leaflet summarizes the most important information about Prezista. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about Prezista that is written for health professionals. For more information, call 1-800-526-7736.
How should I store Prezista?
- Store Prezista oral suspension and tablets at room temperature 77°F (25°C).
- Do not refrigerate or freeze Prezista oral suspension.
- Keep Prezista oral suspension away from high heat.
- Prezista oral suspension should be stored in the original container.
Keep Prezista and all medicines out of the reach of children.
What are the ingredients in Prezista?
Active ingredient: darunavir
Inactive ingredients:|
Prezista oral suspension: citric acid monohydrate, hydrochloric acid (for pH adjustment), hydroxypropyl cellulose, masking flavor, methylparaben sodium, microcrystalline cellulose, purified water, sodium carboxymethylcellulose, strawberry cream flavor, and sucralose.
Prezista 75 mg and 150 mg tablets: colloidal silicon dioxide, crospovidone, magnesium stearate, microcrystalline cellulose. The film coating contains: Opadry White (polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, titanium dioxide).
Prezista 600 mg tablets: colloidal silicon dioxide, crospovidone, magnesium stearate, microcrystalline cellulose. The film coating contains: Opadry Orange (FD&C Yellow No. 6, polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, titanium dioxide).
Prezista 800 mg tablets: colloidal silicon dioxide, crospovidone, magnesium stearate, microcrystalline cellulose, hypromellose. The film coating contains: Opadry Dark Red (iron oxide red, polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, titanium dioxide).
Instructions for use for Prezista oral suspension
Be sure that you read, understand, and follow these Instructions for Use so that you measure and take Prezista oral suspension correctly. Ask your healthcare provider if you are not sure.
Each Prezista oral suspension carton contains:
- 1 bottle of Prezista oral suspension
- Oral dosing syringe
- 1 oral syringe adapter

Important information for use:
Shake Prezista oral suspension well before each use.
Prezista oral suspension should be given with the oral dosing syringe provided to make sure you measure the right amount. The oral dosing syringe provided with your Prezista oral suspension should not be used for any other medicines.
1. Shake the bottle.
- Shake the bottle well before each use (See Figure A).
Figure A
2. Open the bottle of Prezista oral suspension.
- Open the bottle by pushing downward on the cap and twisting it in the direction of the arrow (counter-clockwise) (See Figure B).
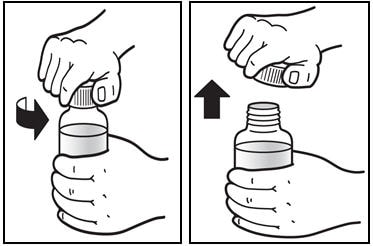
Figure B
3. The first time a bottle of Prezista oral suspension is used:
- Insert the oral syringe adapter into the bottle. Press on the adapter until it is even with the top of the bottle (See Figure C).
- Do not remove the oral syringe adapter from the bottle once inserted.
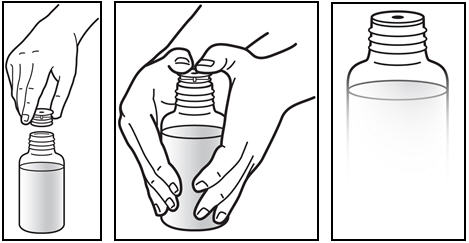
Figure C
4. Insert the oral dosing syringe.
- Fully push down (depress) the plunger of the syringe.
- Insert the syringe into the opening of the oral syringe adapter until it is firmly in place (See Figure D).
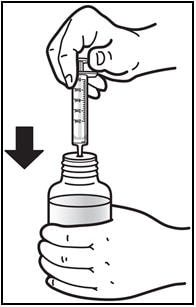
Figure D
5. Withdraw the prescribed amount of Prezista oral suspension.
- Turn the bottle upside down. Gently pull back the plunger of the syringe until the bottom of the plunger is even with the markings on the syringe for the prescribed dose (See Figure E). If you see air bubbles in the syringe, push the plunger in to empty the oral suspension back into the bottle. Then repeat steps 4 and 5.
- If you or your child's dose of Prezista oral suspension is more than 6 mL, you will need to divide the dose. Follow the instructions given to you by your healthcare provider or pharmacist about how to divide the dose.
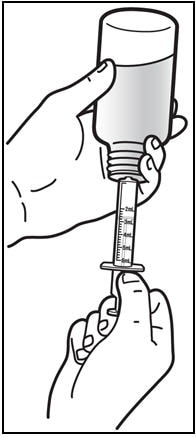
Figure E
6. Removing the syringe
- Turn the bottle upright and remove the syringe from the bottle by pulling straight up on the oral dosing syringe (See Figure F).
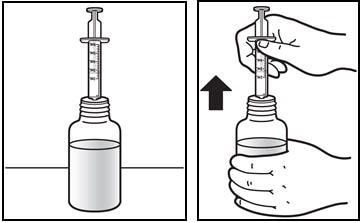
Figure F
7. Take the dose of Prezista.
- Place the tip of the oral dosing syringe in the mouth.
- Press on the plunger of the syringe towards the mouth (See Figure G).
If you or your child's dose is more than 6 mL you will need to divide the dose. Follow the instructions given to you by your healthcare provider or pharmacist about how to divide the dose, and repeat steps 4 through 7.
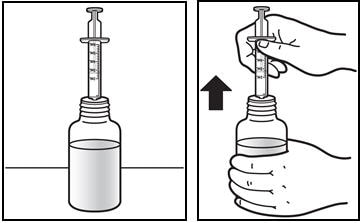
Figure G
8. Close the bottle with the cap after use.
Close the bottle by twisting the cap in the direction of the arrow (clockwise) (See Figure H).
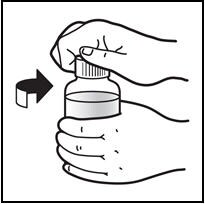
Figure H
9. Remove the plunger from the barrel.
- Remove the plunger from the barrel by pulling the plunger and barrel away from each other (See Figure I). Rinse both parts of the syringe with water and allow to air dry after each use.
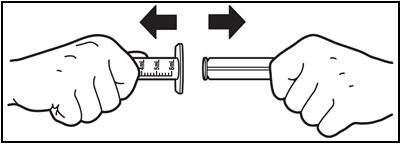
Figure I
10. Putting the syringe back together.
- After air drying, put the oral dosing syringe back together by inserting the plunger into the barrel (See Figure J). Store the oral dosing syringe with Prezista oral suspension.
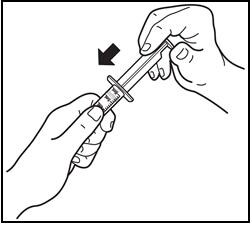
Figure J
How should I store Prezista?
- Store Prezista oral suspension and the oral dosing syringe at room temperature 77°F (25°C).
- Do not refrigerate or freeze Prezista oral suspension.
- Keep Prezista oral suspension away from high heat.
- Store Prezista oral suspension in the original container.
Keep Prezista and all medicines out of the reach of children.
Instructions for use approved 01/2018.