What is Trushesa?
Trushesa is a prescription medicine used for the acute treatment of migraine with or without aura in adults.
- Trushesa is not used to prevent migraine.
- Trushesa is not used to treat other types of headaches such as hemiplegic (that make you unable to move on one side of your body) or basilar (rare form of migraine with aura) migraines.
It is not known if Trushesa is safe and effective in children.
What is the most important information I should know about Trushesa?
Trushesa can cause serious side effects, including:
- Serious problems with blood circulation to your legs and feet (peripheral ischemia). Trushesa can cause peripheral ischemia when you take it with certain medicines known as CYP3A4 inhibitors. Peripheral ischemia may lead to a stroke and death. Stop taking Trushesa and get emergency medical help right away if you have any of the following symptoms:
- cramping and pain in your legs or hips
- feeling of heaviness or tightness in your leg muscles
- burning or aching pain in your feet or toes while resting
- numbness, tingling, or weakness in your legs
- cold feeling or color changes in 1 or both legs or feet
- slurred speech
- sudden weakness
Do not take medicines known as strong CYP3A4 inhibitors, such as:- ritonavir
- nelfinavir
- erythromycin
- clarithromycin
- ketoconazole
- itraconazole
These are not all of the medicines that could affect how Trushesa works. Your healthcare provider can tell you if it is safe to take Trushesa with other medicines.
Who should not use Trushesa?
Do not take Trushesa if you:
- are taking medicines known as strong CYP3A4 inhibitors.
- have heart problems or a history of heart problems.
- have uncontrolled high blood pressure.
- have narrowing of blood vessels in your legs, arms, stomach, or kidneys (peripheral vascular disease).
- have sepsis.
- have had vascular surgery.
- have severe liver problems.
- have severe kidney problems.
- are allergic to dihydroergotamine mesylate, ergot alkaloids, or any ingredients in Trushesa. See the end of this Medication Guide for a complete list of ingredients.
- have taken any of the following medicines in the last 24 hours:
- sumatriptan
- almotriptan
- eletriptan
- frovatriptan
- naratriptan
- rizatriptan
- ergotamine or ergotamine-type medicines
- have taken any medicines that constrict your blood vessels or raise your blood pressure.
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take Trushesa with other medicines.
What should I tell my healthcare provider before using Trushesa?
Before you take Trushesa, tell your healthcare provider about all of your medical conditions, including if you:
- have high blood pressure.
- have liver problems.
- have kidney problems.
- smoke.
- are pregnant or plan to become pregnant. Trushesa may cause preterm labor. Trushesa should be avoided during pregnancy. Talk to your healthcare provider right away if you are pregnant or want to become pregnant.
- are breastfeeding or plan to breastfeed. Trushesa may reduce breast milk supply and pass into your breast milk. Trushesa may be harmful to your baby. Do not breastfeed your baby while taking Trushesa and for 3 days after you use Trushesa. Talk with your healthcare provider about the best way to feed your baby if you take Trushesa.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Your healthcare provider will decide if you can take Trushesa with your other medicines.
Especially tell your healthcare provider if you take:
- sumatriptan
- ergot-type medicine
- saquinavir
- nefazodone
- fluconazole
- grapefruit juice
- zileuton
- nicotine
- propranolol or other medicines that can lower your heart rate
- any medicines that can increase your blood pressure
- selective serotonin reuptake inhibitors
These are not all of the medicines that could affect how Trushesa works. Your healthcare provider can tell you if it is safe to take Trushesa with other medicines.
How should I use Trushesa?
- Certain people should take their first dose of Trushesa in their doctor's office or in another medical setting. Ask your doctor if you should take your first dose in a medical setting.
- Use Trushesa exactly as your healthcare provider tells you to use it. Read and follow the instructions in the Instructions for Use which is provided with the Trushesa package before using.
- You should use Trushesa as soon as the symptoms of your headache start, but it may be given at any time during a migraine.
- After putting Trushesa together and priming the device, spray 1 time in each nostril (a complete dose).
- If your headache comes back after the first complete dose or you only get some relief from your headache, you can use a second dose 1 hour after the first complete dose. Use a new Trushesa nasal spray device for the second dose.
- Do not use more than 2 doses of Trushesa within a 24-hour period or 3 doses within a 7-day period.
- If you use too much Trushesa, call your healthcare provider or go to the nearest hospital emergency room right away.
- Taking Trushesa for 10 or more days in 1 month may make your headaches worse. You should write down when you have headaches and when you take Trushesa so that you can talk with your healthcare provider about how Trushesa is working for you.
What are the possible side effects of Trushesa?
Trushesa can cause serious side effects, including:
See "What is the most important information I should know about Trushesa?"
- Heart attack and other heart problems. Heart problems may lead to death. Stop taking Trushesa and get emergency medical help right away if you have any of the following symptoms of a heart attack:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
Trushesa is not for people with risk factors for heart disease unless a heart exam is done and shows no problem. You have a higher risk for heart disease if you:- have high blood pressure
- smoke
- have diabetes
- have high cholesterol levels
- are overweight
- have a family history of heart disease
- Stroke. Stop taking Trushesa and get emergency medical help right away if you have any of the following symptoms of a stroke:
- face drooping
- slurred speech
- unusual weakness or numbness
- Changes in color or sensation in your fingers and toes (Raynaud's syndrome).
- Stomach and intestinal problems (gastrointestinal and colonic ischemic events). Symptoms of gastrointestinal and colonic ischemic events include:
- sudden or severe stomach pain
- stomach pain after meals
- weight loss
- nausea or vomiting
- constipation or diarrhea
- bloody diarrhea
- fever
- Increase blood pressure.
- Medicine overuse headache. Some people who use too much Trushesa may make their headaches worse (medicine overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with Trushesa.
- Preterm labor.
- Tissue changes (fibrotic complications). Inflammation and fiber-like tissue that is not normal (fibrosis) can occur around the lungs and stomach.
- Burning feelings in your nose, mouth and throat and abnormal taste.
The most common side effects of Trushesa include:
- runny nose
- nausea
- abnormal taste
- application site reactions
- dizziness
- vomiting
- sleepiness
- sore throat
- diarrhea
These are not all the possible side effects Trushesa.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Trushesa
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Trushesa for a condition for which it was not prescribed. Do not give Trushesa to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Trushesa that is written for health professionals.
How should I store Trushesa?
Keep Trushesa away from heat and light.
- Store Trushesa at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not refrigerate or freeze.
- After a Trushesa vial has been opened, it must be thrown away after 8 hours.
Keep Trushesa and all medicines out of the reach of children.
Do not throw Trushesa into fire or incinerators as the canister inside the device may explode.
What are the ingredients in Trudhesa?
Active ingredient: Dihydroergotamine mesylate
Inactive ingredients: Caffeine, carbon dioxide, dextrose, and water. The nasal spray device canister contains hydrofluoroalkane-134a (HFA) propellant. The vial stopper is not made with natural rubber latex.
For more information, go to www.trudhesa.com or call 1-833-878-3437.
Instructions for use for Trudhesa
Trudhesa
(true-deh-sa)
(dihydroergotamine mesylate)
nasal spray
For Nasal Use Only
This Instructions for Use contains information on how to deliver Trudhesa.
Introduction
Read this Instructions for Use before you start to use Trudhesa and each time you get a prescription refill. There may be new information.
This information does not take the place of talking with your healthcare provider about your medical condition or treatment. You and your healthcare provider should talk about Trudhesa when you start taking it and at regular checkups.
It is important to follow these directions accurately in order to receive the correct dose. Contact your healthcare provider if you have any questions about how to use this product.
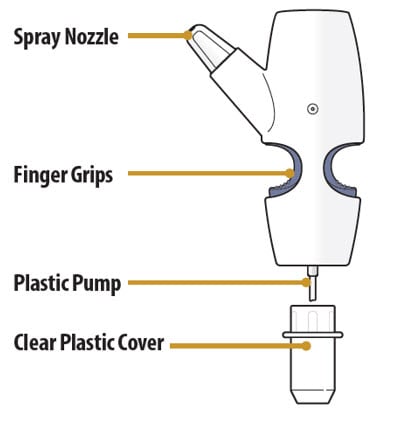
Nasal Spray Device Parts
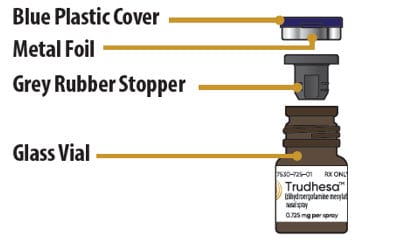
Glass Vial Parts
Important Information You Need To Know Before Dosing with Trudhesa
- For nasal use only.
- Always prime the nasal spray device before dosing by pumping the finger grips and glass vial together exactly 4 times.
- The purpose of priming is to bring the medicine to the tip of the spray nozzle. You may or may not see liquid or spray come out of the nozzle during each priming action.
- During priming, make sure to aim the spray nozzle away from your face and anything that you do not want coming into contact with the spray of medicine.
- A complete dose is 2 sprays; 1 spray in each nostril.
- Do not take more than 2 doses within a 24-hour period. Do not take more than 3 doses in a 7-day period.
- Always hold the nasal spray device perfectly upright when priming and when dosing.
- Sniffing while dosing is not necessary. However, sniffing will not hurt you nor make the medicine less effective.
- This nasal spray device product is single-dose (for one complete dose only) and should be thrown away (discarded) after use. You will need a new kit for each dose.
- Keep the product in the case until ready to use.
- After a Trudhesa vial has been opened, it must be thrown away after 8 hours.
- Do not open the glass vial and expose to air until ready to use.
- Store at room temperature in a clean, dry area.
- Do not use if product is damaged.
- Do not use if product is expired.
- Each glass vial and nasal spray device can only be used 1 time. Throw away the entire nasal spray device after dosing, without removing the glass vial.
- You can take another complete dose at least 1 hour after your first dose if your symptoms persist
Storing Trudhesa
- Store Trudhesa at room temperature between 68 °F to 77°F (20 °C to 25°C).
- Store Trudhesa in the original packaging in a clean, dry area away from heat and light (Figure A).
- Keep Trudhesa in the original packaging until you are ready to use.
- Do not refrigerate or freeze Trudhesa.
- Keep Trudhesa and all medicines out of the reach of children.
Figure A
Preparing to Dose with Trudhesa
Step 1: Gather and Check Supplies
1.1. Check to make sure you are using the right medicine for your migraine (see Figure B).
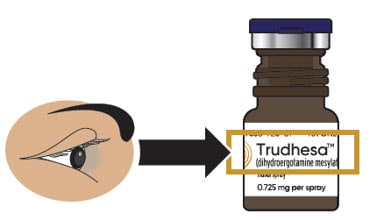
Figure B
1.2 Check to make sure Trudhesa is not expired (EXP) (see Figure C).
- If expired, throw away and get a new glass vial.
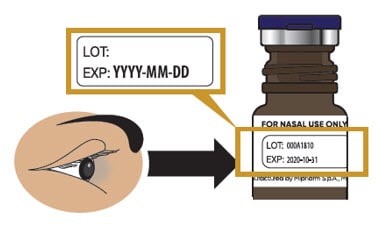
Figure C
1.3 Check to make sure that the glass vial and blue plastic cover do not look damaged.
Step 2: Remove Blue Plastic Cover, Metal Foil and Grey Rubber Stopper from Glass Vial
2.1 Remove (flip up) the blue plastic cover from the glass vial (see Figure D).
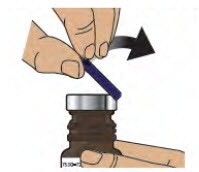
Figure D
2.2. Use the blue plastic cover to slowly peel away all the metal foil from the grey rubber stopper in a circular motion (see Figure E).
NOTE: The metal foil may come off in 2 or more pieces. Make sure to remove all of the metal foil.
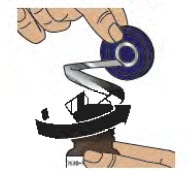
Figure E
2.3 Pull the grey rubber stopper up and out of the glass vial (Figure F and Figure G).
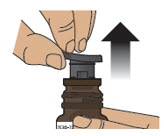
Figure F

Figure G
2.4 Throw away (discard) cover, foil and grey rubber stopper into the trash.
Step 3: Remove the Clear Plastic Cover from the Nasal Spray Device
3.1 Hold the nasal spray device upright.
3.2 Pull down on the clear plastic cover and remove it from the nasal spray device (Figure H).
- Throw away the clear plastic cover.
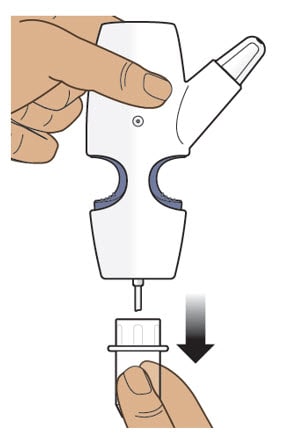
Figure H
Step 4: Screw the Glass Vial into the Nasal Spray Device
4.1 Hold the nasal spray device upright.
4.2 Gently push glass vial into the bottom of the nasal spray device (see Figure I) and screw it on until it is secured as shown in Figure J.
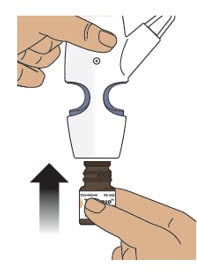
Figure I
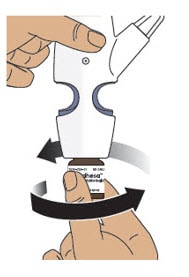
Figure J
Step 5: Prime the Nasal Spray Device by Pumping Four Times with Fin and Thumb
5.1 Hold the nasal spray device upright.
5.2 Point the spray nozzle away from your face.
5.3 Place your thumb on the bottom of the glass vial and place your pointer (index) and middle fingers on the finger grips (see Figure K).
5.4 Pump the nasal spray device exactly 4 times.
- To pump the nasal spray device, firmly press the finger grips down and press the glass vial up at the same time. Then release (see Figure K).
- You may see some medicine spray out during priming. This is normal. It is okay if you do not see medicine spray out on the first few pumps.
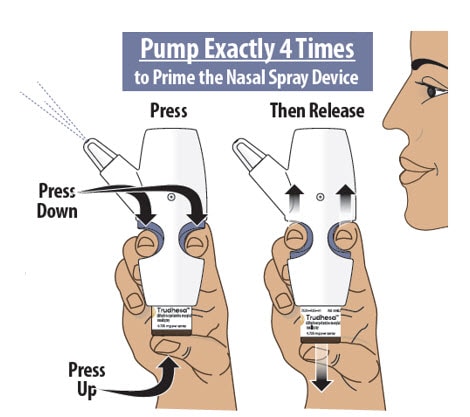
Figure K
You may or may not see liquid each time you pump.
Only pump 4 times to prime.
Important Tip: The purpose of priming is to bring the medicine to the tip of the spray nozzle. If you do not prime the nasal spray device, you will not get your correct dose of medicine.
Always prime the nasal spray device before use by pumping exactly 4 times.
During priming, make sure to aim the nozzle away from your face and anything that you do not want coming into contact with the spray of medicine.
Using Trudhesa
Step 6: Position the Nasal Spray Device
6.1 Turn or re-grip the nasal spray device so that the spray nozzle faces you.
6.2 Make sure your head is straight and the nasal spray device is upright.
6.3 Insert the spray nozzle into your first nostril as far as comfortable (see Figure L).
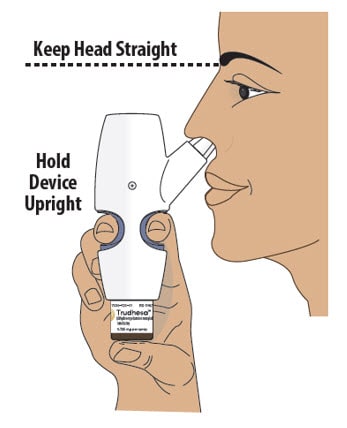
Figure L
Step 7: Spray the First Spray into 1 Nostril
7.1 Firmly press the finger grips down and press the glass vial up at the same time to deliver the first spray (see Figure M). Then release.
- Only deliver 1 spray into this nostril.
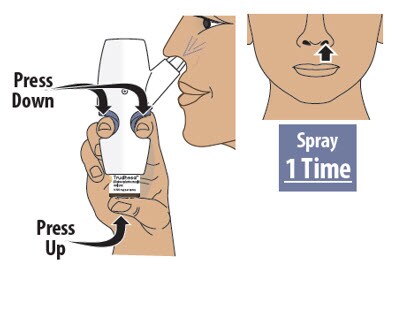
Figure M
Step 8: Spray the Second Spray into Other Nostril
8.1 Move the spray nozzule into your other nostril.
8.2 Firmly press the finger grips down and press the glass vial up at the same time to deliver the second spray (see Figure N). Then release.
- Only deliver 1 spray into this nostril.
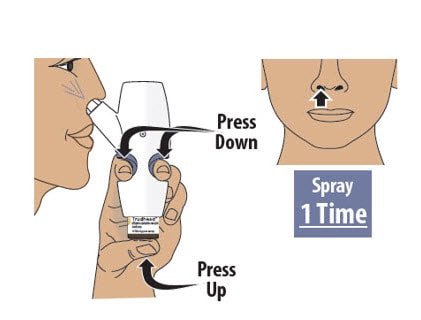
Figure N
Important Tip: A complete dose is 2 sprays; 1 spray in each nostril.
Do not take more than 2 doses within a 24-hour period. Do not take more than 3 doses in a 7-day period. Please refer to the prescribing information for more information.
Sniffing while or after dosing is not necessary. However, sniffing will not hurt you or make the medicine less effective.
Step 9: Throw away Trudhesa
9.1. Throw away the entire nasal spray device, with the glass vial attached, into your household trash (Figure O).
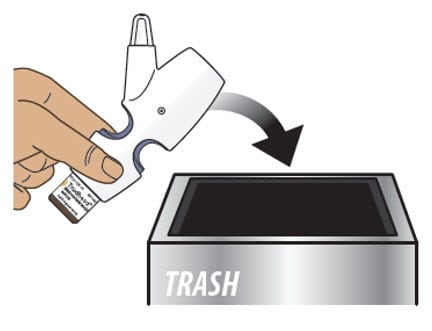
Figure O
- Do not separate the glass vial from the nasal spray device.
- Do not reuse the glass vial or the nasal spray device. The glass vial and nasal spray device can only be used 1 time.
- Do not throw Trudhesa into fire or incinerators.
Important Tip: Some medicine may remain in the glass vial. This is normal. Remaining medicine cannot be used for later dosing.
Important Frequently Asked Questions (FAQs)
Question: Can I save medicine by skipping the 4 pumps in "Step 5: Prime the Nasal Spray Device?"
Answer: No. Skipping the 4 pumps to prime the nasal spray device can result in you not getting your correct dose of medicine.
Question: When I first pumped the nasal spray device to prime nothing seemed to happen. Why?
Answer: The purpose of priming is to bring the medicine up to the tip of the nozzle. Even though you may not see or hear anything on your first pump or two, the pumping action will move the medicine from the glass vial through the inside of the nasal spray device and into the nozzle. You should see a spray by your fourth pump attempt. Always prime by pumping exactly 4 times prior to dosing.
Question: Can I reuse the nasal spray device with a new glass vial?
Answer: No. The nasal spray device is for one-time use only and must be thrown away after you have dosed (1 spray in each nostril). This is because the device may clog. After dosing, leave the glass vial screwed onto the nasal spray device and throw away the assembled nasal spray device into the trash. Do not recycle any part of the product.
Question: Can I use the medicine that remains in the glass vial for a later dose?
Answer: No. Although it is normal for some medicine to remain in the glass vial, it can not be used for later dosing. Any leftover medicine will become ineffective.
Question: What happens if I spray more than one time in the same nostril?
Answer: A complete and correct dose is one spray into each nostril. Do not dose in your other nostril if you already sprayed two times in one nostril. Talk to your healthcare provider if you have moderate to severe irritation in your nose or changes in smell.
Question: How soon can I take another dose if I am not getting relief from my migraine?
Answer: You can take another dose at least 1 hour after your first dose if your symptoms persist. Do not take more than 2 doses within a 24-hour period. Do not take more than 3 doses in a 7-day period. Please refer to the prescribing information for more information.
Instructions for use issued 09/2021




