What is Twirla?
Twirla is a birth control patch for women with a Body Mass Index (BMI) less than 30 kg/m2 who can become pregnant. It contains two female hormones, a progestin called levonorgestrel, and an estrogen called ethinyl estradiol. Birth control methods that have both an estrogen and a progestin are called combination hormonal contraceptives (CHCs).
Twirla is less effective in women with a BMI of 25 kg/m2 or more.
How well does Twirla work?
Your chance of getting pregnant depends on how well you follow the directions for using Twirla. The better you follow the directions, the less chance you have of getting pregnant.
For Twirla to be most effective, you must use Twirla exactly as your healthcare provider tells you to. Each patch must be fully attached to the skin during the 7 days in order for it to work the best.
Twirla is less effective in women who have a BMI of 25 kg/m2 or more. If you have a BMI of 30 kg/m2 or more, talk to your healthcare provider about other methods of birth control which may be right for you.
What is the most important information I should know about Twirla?
- Do not use Twirla if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects from combination hormonal contraceptives (CHCs), including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
- Do not use Twirla if your BMI is 30 kg/m2 or more. If you do not know what your BMI is, please talk to your health care provider. Women with a BMI of 30 kg/m2 or more who use CHCs may have a higher risk for developing side effects like blood clots compared to women with a BMI lower than 30 kg/m2.
Hormonal birth control methods help to lower the chances of becoming pregnant when taken as directed. Twirla does not protect against HIV infection (AIDS) and other sexually transmitted infections (STIs).
Who should not use Twirla?
Do not use Twirla if you:
- smoke and are over 35 years old.
- have or have had blood clots in your arms, legs, eyes or lungs.
- have had a stroke.
- have had a heart attack.
- have certain heart valve problems or heart rhythm problems that can cause blood clots to form in the heart.
- have a problem that makes your blood clot more than normal that you were born with (inherited) or that has happened for other reasons such as medicines, surgery or injuries (acquired).
- have high blood pressure that is not controlled.
- have diabetes and you are over the age of 35, have high blood pressure or have kidney, eye, nerve, or blood vessel damage, or have had diabetes for more than 20 years.
- have had certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision, or have any migraine headaches if you are over age 35.
- have a BMI of 30 kg/m2 or more.
- have liver problems including liver tumors, hepatitis, cirrhosis, or liver disease.
- have unexplained vaginal bleeding.
- are pregnant or think you may be pregnant. However, Twirla is not known to cause birth defects when used by accident during pregnancy.
- have had breast cancer or any cancer that is sensitive to female hormones.
- are allergic to any of the ingredients in Twirla. See a complete list of ingredients at the end of this Patient Information guide. Symptoms of an allergic reaction you may include itching and irritation at the patch site.
- take any Hepatitis C drug combination containing ombitasvir, paritaprevir, ritonavir, with or without dasabuvir. This may increase levels of a liver enzyme called alanine aminotransferase (ALT) in the blood.
Twirla may not be a good choice for you if you have ever had jaundice (yellowing of the skin or eyes) caused by pregnancy (also called cholestasis of pregnancy) or related to previous use of hormonal birth control.
Tell your healthcare provider if you have ever had any of the above conditions. Your healthcare provider may recommend another method of birth control.
What should I tell my healthcare provider before using Twirla?
Before using Twirla, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or think you are pregnant. Twirla is not for pregnant women. If you think you are pregnant, you should have a pregnancy test and know the results. Do not use Twirla if the test is positive and talk to your healthcare provider.
- are scheduled for surgery. Twirla may increase your risk of blood clots after surgery. You should stop using your Twirla patch at least 4 weeks before you have surgery and not restart it until at least 2 weeks after your surgery.
- have or have had gallbladder problems including yellowing of the skin or eyes during pregnancy.
- have high cholesterol that is not controlled.
- have or have had depression.
- have a history of hereditary angioedema.
- have had dark patches of skin on your forehead, cheeks, upper lip, and chin (chloasma).
- are breastfeeding or plan to breastfeed. CHC medicines that contain estrogen, like Twirla, may decrease the amount of milk you make. A small amount of hormones from the Twirla patch may pass into your breast milk. You may want to use another method of birth control until you are ready to stop breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines and herbal products may make Twirla less effective or cause breakthrough bleeding, including, but not limited to:
- certain anti-seizure medicines (such as barbiturates, carbamazepine, felbamate, oxcarbazepine, phenytoin, rufinamide, or topiramate).
- medicine to treat chemotherapy-induced nausea and vomiting (aprepitant).
- medicine to treat high blood pressure in the vessels of the lung (bosentan).
- a certain medicine used to treat fungal infections (griseofulvin).
- certain combinations of HIV medicines (nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/rotinavir, and tipranavir/ritonavir).
- certain non-nucleoside reverse transcriptase inhibitors (such as nevirapine and efavirenz).
- rifampin and rifabutin.
- certain hepatitis C (HCV) medicines (such as boceprevir, telaprevir).
- St. John’s wort.
- Use another birth control method (such as condoms and spermicide, or diaphragm and spermicide) when you take medicines that may make Twirla less effective and for 28 days after stopping the medicine.
- Some medicines and grapefruit juice may increase your level of the hormone ethinyl estradiol if used together, including:
- the pain reliever acetaminophen.
- ascorbic acid (vitamin C).
- certain medicines used to treat fungal infections (itraconazole, ketoconazole, voriconazole, and fluconazole).
- certain HIV medicines (atazanavir/ritonavir, indinavir).
- non-nucleoside reverse transcriptase inhibitors (such as etravirine).
- medicines to lower cholesterol (such as atorvastatin and rosuvastatin).
- Twirla may affect the way that lamotrigine, a medicine used to treat seizures, works and may increase the risk of seizures. Your healthcare provider may need to adjust the dose of lamotrigine while you are on Twirla.
- If you are scheduled for any laboratory tests, tell your healthcare provider that you are using Twirla. Certain blood tests may be affected by CHC methods.
- Women on thyroid replacement therapy may need increased doses of thyroid replacement medicine or corticosteroid replacement medicine may need increased doses of their thyroid hormone or cortisol medicines.
Ask your healthcare provider if you are not sure if you take any of the medicines listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. Talk to your healthcare provider before you start taking a new medicine.
How should I use Twirla?
- For detailed instructions, see the step-by-step instructions for using Twirla at the end of this Patient Information leaflet.
- Use Twirla exactly as your healthcare provider tells you to use it. Wear 1 Twirla patch at a time.
- Do not skip using any Twirla patches, even if you do not have sex often.
- Twirla is applied in a 4 week patch cycle. Each patch cycle includes 4 weeks (28 days). You will put on 1 patch every week for 3 weeks. You will not wear a patch during week 4. Each patch is worn for 7 days (1 week).
- Apply a new Twirla patch on the same day each week (this is called your Patch Change Day). For example, if you apply your first patch on a Monday, all of your Twirla patches should be applied on Monday.
- You will not wear a Twirla patch during week 4 (this is called your Patch Free Week). Make sure you remove the old patch from your body. Your period should begin during your Patch Free Week. After you have finished week 4, apply a new Twirla patch on the day after Week 4 ends. Repeat the patch cycle of 1 patch a week for 3 weeks followed by your Patch Free Week.
- Do not cut, damage or change the Twirla patch in any way. If the patch is cut, damaged or changed in any way, it may be less effective.
- Your Twirla patch should never be off more than 7 days in a row. If you ever go more than 7 days without a patch, you should use another non-hormonal back up birth control method.
- If you miss your Patch Change Day, put the patch on late or if it comes off of your skin before your Patch Change Day, you may or may not need to use another non-hormonal back up birth control method. See the detailed table in the Instructions for Use for more information.
- If you miss a period you might be pregnant. Some women miss their periods or have light periods on hormonal birth control methods even when they are not pregnant. Call your healthcare provider if you miss 1 period and have not used your Twirla patch every day or you miss 2 periods in row.
What should I avoid while using Twirla?
- Smoking
- The following can cause the patch to not stick the right way making Twirla less effective:
- Avoid using makeup, creams, lotions, oils, powders or any other products on the skin area where you put or plan to put the patch.
- Swimming or contact with water often or for long periods of time (30 minutes or more). Talk with your healthcare provider about the best method of birth control if you are a swimmer or you often come in contact with water for 30 minutes or more.
- Women who tend to get chloasma should avoid spending a long time in sunlight, tanning booths, and under sun lamps while using Twirla. Use sunscreen if you have to be in the sunlight.
What are the possible side effects of Twirla?
Twirla may cause serious side effects, including:
- See “What is the most important information I should know about Twirla?”
- blood clots. Like pregnancy, hormonal birth control may increase the risk of serious blood clots (see following graph), especially in women who have other risk factors, such as smoking, high blood pressure, high levels of fat in the blood, diabetes, obesity, a family history of blood clots or are older than 35 years old. This increased risk is highest when you first start using hormonal birth control and when you restart the same or different hormonal birth control after not using it for a month or more. Some studies have reported that women who use levonorgestrel and ethinyl estradiol transdermal system have a higher risk of getting a blood clot. Talk to your healthcare provider about your risk of getting a blood clot before using Twirla or deciding which type of birth control is right for you.
It is possible to die or be permanently disabled from a problem caused by a blood clot, such as a heart attack or a stroke. Some examples of serious blood clots are blood clots in the:- legs (deep vein thrombosis)
- lungs (pulmonary embolus)
- eyes (loss of eyesight)
- heart (heart attack)
- brain (stroke)
- To put the risk of developing a blood clot into perspective: If 10,000 women who are not pregnant and do not use hormonal birth control are followed for one year, between 1 and 5 of these women will develop a blood clot. The figure below shows the likelihood of developing a serious blood clot for women who are not pregnant and do not use hormonal birth control, for women who use hormonal birth control, for pregnant women, and for women in the first 12 weeks after delivering a baby.
Likelihood of Developing A Serious Blood Clot (Venous Thromboembolism (VTE))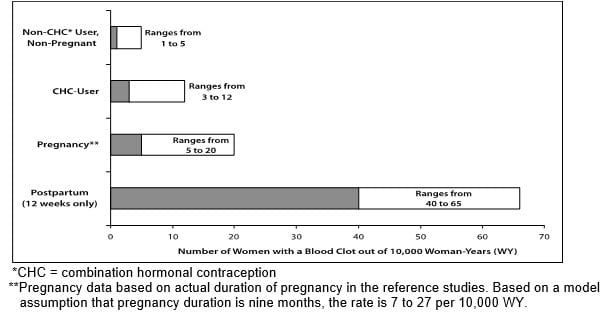
- Call your healthcare provider right away if you have:
- leg pain that does not go away
- sudden shortness of breath
- sudden changes to your vision or blindness
- severe pain or pressure in your chest
- sudden, severe headache unlike your usual headaches
- weakness or numbness in an arm or leg
- trouble speaking
- Call your healthcare provider right away if you have:
- liver problems, including liver tumors. Stop using Twirla and tell your healthcare provider right away if you have yellowing of your skin or eyes (jaundice).
- high blood pressure. Your healthcare provider will check your blood pressure and may stop you from using Twirla if your blood pressure rises.
- gallbladder problems or worsening of a gallbladder problem you already have. You may have an increased risk of gallbladder problems with the use of Twirla especially if you have had gallbladder problems before or gallbladder problems when you were pregnant.
- headaches. Headaches can be a common but serious side effect. Tell your healthcare provider if you have new headaches that keep coming back, that do not go away, or are severe. Also tell your healthcare provider if your migraine headaches happen more often or are more severe than normal. Your healthcare provider may stop you from using Twirla.
- irregular or unusual vaginal bleeding and spotting between your menstrual periods or absence of menstrual periods (amenorrhea). This can happen especially during the first 3 months of using Twirla. You also may have no bleeding at all. Tell your healthcare provider if you miss 2 or more menstrual cycles. After you stop using Twirla, your periods may not happen as often or you may have no bleeding at all, especially if you had these types of menstrual cycles before taking Twirla.
- depression.
- swelling of your skin especially around your mouth, eyes, and in your throat (angioedema). Call your healthcare provider or get emergency medical care right away if you have a swollen face, lips, mouth, tongue or throat as this may lead to difficulty swallowing or breathing. Your risk of having angioedema is higher if you have a history of angioedema.
- dark patches of skin on your forehead, cheeks, upper lip, and chin (chloasma). Your risk of getting chloasma with the use of Twirla is higher if you had chloasma during pregnancy.
The most common side effects of Twirla include:
- skin reactions at the patch site such as bumps, redness or changes in color of your skin, bleeding itching, rash, dryness, pain and swelling.
- headache
- weight gain
- nausea
- menstrual cramps
These are not all the possible side effects of Twirla.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Does hormonal birth control cause cancer?
Hormonal birth control does not seem to cause breast cancer. However, if you have breast cancer now, or have had it in the past, do not use hormonal birth control because some breast cancers are sensitive to hormones. Women who use hormonal birth control may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as an increased number of sexual partners.
What should I know about my period when using Twirla?
When you use Twirla you may have bleeding and spotting between periods, called unplanned bleeding. Unplanned bleeding may vary from light slight staining between menstrual periods to breakthrough bleeding which is a flow much like a regular period. Unplanned bleeding occurs most often during the first few months of hormonal contraceptive use but may also occur after you have been using the patch for some time. Such bleeding may be temporary and usually does not indicate any serious problems. It is important to continue using the patch on schedule. If the unplanned bleeding or spotting occurs in more cycles, is unusually heavy, or lasts for more than a few days, talk to your healthcare provider.
What if I miss my scheduled period when using Twirla?
You should consider the possibility that you are pregnant if you miss your scheduled period. Because scheduled periods may not happen as often when you are using Twirla, tell your healthcare provider that you have missed your period and that you are using Twirla. Also, notify your healthcare provider if you have symptoms of pregnancy such as morning sickness or unusual breast tenderness. It is important that your healthcare provider checks to see if you are pregnant. Stop using Twirla if you are pregnant.
What if I want to become pregnant?
You may stop using Twirla whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop using Twirla.
Twirla Images
General information about the safe and effective use of Twirla
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Twirla for a condition for which it was not prescribed. Do not give Twirla to other people. It may harm them. You can ask your pharmacist or healthcare provider for information about Twirla that is written for health professionals.
How should I store Twirla?
- Store at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Twirla in the original unopened pouch it comes in. Apply Twirla immediately after taking it out from the pouch.
- Do not store Twirla in the refrigerator or freezer.
- Used Twirla patches may still have some active hormones. To throw away the Twirla patch, fold the sticky side of the patch together, and place this container in the trash. Do not flush used Twirla patches down the toilet.
Keep Twirla and all medicines out of the reach of children.
What are the ingredients in Twirla?
Active ingredients: levonorgestrel (a progestin) and ethinyl estradiol (an estrogen)
Inactive ingredients: polyester release liner, woven polyester backing membrane, acrylic adhesives, polyester internal membrane, polyisobutylene adhesives, copovidone, polybutene, crospovidone, lauryl lactate, dimethyl sulfoxide, capric acid, and ethyl lactate.
Instructions for use for Twirla
Twirla (TWER-la)
(levonorgestrel and ethinyl estradiol) transdermal system
Twirla is for skin use only.
Read this Instructions for Use before you start using the Twirla transdermal system (TDS) (also called a patch) and
each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your contraceptive treatment.
Do not cut, damage or change the Twirla patch in any way. If the patch is cut, damaged or changed in any way, it may be less effective.
Starting Twirla for the first time: If you are starting Twirla for the first time, you should wait until you begin your menstrual period.
- Day 1 Start. You should apply your first patch during the first 24 hours of your menstrual period. Your Patch Change Day will be on this day every week. If you start after Day 1 of your menstrual period, non-hormonal back up birth control (such as condoms and spermicide, or diaphragm and spermicide) should be used in addition to the patch for the first 7 days of your first patch cycle.
If you are changing from the oral hormone birth control pills, vaginal contraceptive ring or another transdermal patch to Twirla:
- Day 1 Start: You should apply your first patch during the first 24 hours of your menstrual period. Your Patch Change Day will be on this day every week. If you start after Day 1 of your menstrual period, non-hormonal back up birth control (such as condoms and spermicide, or diaphragm and spermicide) should be used in addition to the patch for the first 7 days of your first patch cycle.
- Finish your current oral hormone birth control pill cycle, vaginal ring cycle or other transdermal patch cycle. Apply your first Twirla patch on the day you would normally start your next oral birth control pill, patch or insert your next vaginal ring.
- If you do not get your period within 1 week after taking your last active oral hormone birth control pill, removing your last vaginal ring or other transdermal patch cycle, check with your healthcare provider to make sure you are not pregnant. You may still go ahead and start Twirla for contraception.
- If you apply your Twirla patch more than 1 week after taking your last active oral hormone birth control pill, removing your last vaginal ring or other transdermal patch cycle use a non-hormonal contraceptive method with the Twirla patch for the first 7 days of using the patch.
If you are starting Twirla after a miscarriage or abortion:
- You may start Twirla right away after a miscarriage or abortion that occurs in the first 12 weeks (first trimester) of pregnancy. If you start Twirla within 5 days of your first trimester abortion or miscarriage, you do not need to use another back up contraceptive method.
- If you do not start Twirla within 5 days after a first trimester miscarriage or abortion, use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, while you wait for your period to start.
- If you are starting Twirla after a miscarriage or abortion that occurs after the first 12 weeks of pregnancy (second trimester), wait 4 weeks before using Twirla and use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, for the first 7 days of your first patch cycle only.
If you are starting Twirla after childbirth:
- If you are not breastfeeding, wait 4 weeks before using Twirla and use a non-hormonal contraceptive method of birth control, such as a condom and spermicide or diaphragm and spermicide, for the first 7 days of your first patch cycle only. If you start using Twirla after childbirth and have not had your menstrual period, tell your healthcare provider. They will need to make sure you are not ovulating or pregnant before starting Twirla. If your healthcare provider tells you are not pregnant, use a non-hormonal contraceptive method for the first 7 days of patch of your first patch cycle.
How to Apply Twirla:
Where should the patch be applied?
- Wear only 1 patch at a time.
- Before applying the patch, make sure your skin is clean and dry.
- Avoid using make up, creams, lotions, oils, powders or any other products on the skin area where you put or plan to put the patch.
- Application sites to apply the patch include the lower stomach area (abdomen), buttock, or the upper torso.
- When you put the patch on, it should lay flat and smooth with no wrinkles or folds.
- On Patch Change Day, remove the current patch and immediately put on a new patch. Do not apply the new patch directly over skin where the old patch site was. You should use a new application site.
Where not to place the patch.
- Do not put the patch on your waistline or near clothing or undergarment seams.
- Do not put the patch on the breasts, on cut or irritated skin (rashes or other skin problems), or on the same location as the old patch.
Patch Application Instructions
- Before the patch is applied, make sure your skin is clean and dry. Also make sure you have not used any make up, creams, lotions, oils, powders or any other products on the skin area where you put or plan to put the patch
- Each patch is individually sealed in a pouch.
- It is important that you immediately apply the patch after being removed from the pouch.
Step 1. Tear the pouch open at the notch on the pouch.
Step 2. Open the pouch and carefully remove the patch. The patch is attached to a clear protective liner.
Step 3a. Hold the patch with the clear protective liner facing you. You will see two sections: a large section and a small section.
Avoid touching the sticky side of the patch with your fingers.
Step 3b. Hold the small section of the liner. Remove and throw away (discard) the large section of the liner while still holding the small section of the liner.
Step 4. Hold the small section of the liner and apply the sticky side of the patch to the chosen patch site.
Step 5. Press the sticky side of the patch firmly onto your skin and smooth it down.
Step 6. If the patch is not flat on the skin or there are large wrinkles, gently pull the patch off the skin while holding only the remaining protective liner and then put it on again.
Avoid wrinkles or folds.
Step 7. After the patch is flat with no wrinkles, pull an edge of the remaining protective liner and gently pull it off.
Step 8a. After the patch is on your body, press the entire patch firmly into place with your hand for 10 seconds, making sure the edges stick well.
Step 8b. Make sure the patch is on your skin all the way.
Step 9. The edges of the patch should be smoothed over with your finger and make sure there is good contact around the patch with your skin and make sure there are no wrinkles.
Step 10. It is important that you check the patch every day to make sure it is in the right place. The patch should be checked after any water exposure (such as bathing, showering, or swimming) to make sure it is in the right place because water may affect how well the patch sticks to your skin.
How do I throw away Twirla patches?
- To throw away your Twirla patch, fold the sticky side of the patch together and place in the trash right away so that children and pets cannot reach it. Do not flush used Twirla patches down the toilet.
- For more information on how to safely throw away medicines, see www.fda.gov/drugdisposal
When should I change the Twirla patch?
- Twirla is applied in a 4 week patch cycle. Each patch cycle includes 4 weeks (28 days). You will put on 1 patch every week for 3 weeks. You will not wear a patch during week 4. Each patch is worn for 7 days (1 week).
- Apply a new Twirla patch on the same day each week (this is called your Patch Change Day). For example, if you apply your first patch on a Monday, all of your Twirla patches should be applied on Monday.
- You will not wear a Twirla patch during week 4 (this is called your Patch Free Week). Make sure you remove the old patch from your body. Your period should begin during your Patch Free Week.
- After you have finished week 4, apply a new Twirla patch on the day after Week 4 ends. Repeat the patch cycle of 1 patch a week for 3 weeks followed by your Patch Free Week.
What if a patch starts to lift off your skin or completely comes off?
- If your patch starts to lift off your skin or completely comes off and you do not replace it, you may not get enough hormones to keep you from getting pregnant.
- If a patch starts to lift off your skin or is completely off for less than one day (up to 24 hours), you should try and put it on again to the same place or replace it with a new patch immediately. No back up birth control is needed and your Patch Change Day will remain the same.
- If a patch starts to lift off your skin or is completely off for more than 1 day (24 hours or more) or if you are not sure how long the patch has been not attached to your skin, you may not be protected from pregnancy. You should stop your current patch cycle and start over on a new patch cycle right away by putting on a new patch. The day you apply your new patch is now your new Day 1 and your new Patch Change Day. Non-hormonal back up birth control, (such as condoms and spermicide, or diaphragm and spermicide) must be used for the first week of the new patch cycle.
- Do not put a patch on again if it is no longer sticky, if it has become stuck to itself or another surface or if it has other material stuck to it. If your patch cannot be put on again, a new patch should be put on right away. If you need help applying a patch, contact Agile Medical Information at 1-855-389-4752 or email: medicalaffairs@agiletherapeutics.com.
Can I wear the patch when I am exercising, or using a sauna, swimming pool, or whirlpool?
- Yes, women can maintain all their normal daily activities while using the patch.
- It is important to check your patch after any water that touches your patch during bathing, showering, or swimming, as prolonged water exposure may affect how well the patch sticks to your skin.
- If the patch starts to come off or completely lifts off the skin, try to put it on again.
- A patch should not be put on again if it is no longer sticky, if it has become stuck to itself or another surface or if it has other material stuck to it.
- If your current patch cannot be put on again, a new patch should be put on right away Before applying the patch, make sure your skin is clean and dry.
- Make sure you have not used any make up, creams, lotions, oils, powders or any other products on the skin area where you put or plan to put the patch. If you find yourself in need of an additional patch because you needed to replace a patch, contact Agile Medical Information at 1-855-389-4752 or email: medicalaffairs@agiletherapeutics.com.
What if you forget to change your patch (left your patch on more than 7 days)?
- If you forget to change your patch at the start of any patch cycle (Day 1): You may not be protected from pregnancy. You should apply the first patch of your new patch cycle as soon as you remember. This is now your new Patch Change Day and your new Day 1. You must use non-hormonal back up birth control (such as condoms and spermicide, or diaphragm and spermicide) for the first week of your new patch cycle.
- If you forget to change your patch in the middle of the patch cycle (Day 8 or Day 15): for 1 or 2 days (up to 48 hours): you should apply a new patch right away. The next patch should be applied on your usual Patch Change Day. No back up birth control is needed.
- If you forget to change your patch for more than 2 days (48 hours or more): You may not be protected from pregnancy. You should stop your current patch cycle and start a new 4week patch cycle right away by putting on a new patch. This is now your new Patch Change Day and your new Day 1. You must use non-hormonal back up birth control for the first week of your new patch cycle.
What to do if the patch starts to lift or the patch completely comes off from the skin and Late or Missed Patch Applications
| Frequent Patch Situations | Will I have a New Patch Change Day | Will I need to start a New 4 week Patch Cycle | Will I need a backup Birth Control Method |
| Did not apply patch on scheduled Day 1 of new patch cycle | Yes | Yes | Yes (for 7 days) |
| Patch not attached for less than 24 hours | No | No | No |
| Patch not attached for 24 hours or more, or unsure how long | Yes | Yes | Yes (for 7 days) |
| Less than 48 hours late for Patch Change Day (Day 8 or 15) | No | No | No |
| 48 hours or more late for Patch Change Day (Day 8 or 15) | Yes | Yes | Yes (for 7 days) |
| Forgets to remove last patch on Day 22 | No | No | No |
What if you forget to remove your patch for the patch free week?
- Past Day 22: You should take it off as soon as you remember. No other change is needed. You should still start the next patch cycle on the usual Patch Change Day, which is the day after Day 28. No back up birth control is needed.
- Your Twirla patch should never be off more than 7 days in a row. If you ever go more than 7 days without a patch, you should use another birth control method.
- As with all hormonal birth control, the risk of getting pregnant increases with each day you go past the recommended 7day patch free period.
What if you wish to change your Patch Change Day?
- If you want to change your Patch Change Day you should complete your current patch cycle, removing the third patch on the correct day. During the Patch Free Week, you may select an earlier Patch Change Day by applying a new patch on the chosen day.
Your Twirla patch should never be off more than 7 days in a row.
For more information call 1-855-389-4752 or email: medicalaffairs@agiletherapeutics.com.