What is Advair HFA?
- Advair HFA combines the inhaled corticosteroid (ICS) medicine fluticasone propionate and the long-acting beta2-adrenergic agonist (LABA) medicine salmeterol.
- ICS medicines such as fluticasone propionate help to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing problems.
- LABA medicines such as salmeterol help the muscles around the airways in your lungs stay relaxed to prevent symptoms, such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe.
- Advair HFA is not used to relieve sudden breathing problems and will not replace a rescue inhaler.
- It is not known if Advair HFA is safe and effective in children younger than 12 years of age.
- Advair HFA is used for asthma as follows:
- Advair HFA is a prescription medicine used to control symptoms of asthma and to prevent symptoms such as wheezing in adults and adolescents aged 12 years and older.
- Advair HFA contains salmeterol, the same medicine found in Serevent Diskus (salmeterol xinafoate inhalation powder). LABA medicines such as salmeterol when used alone increase the risk of hospitalizations and death from asthma problems. Advair HFA contains an ICS and a LABA. When an ICS and LABA are used together, there is not a significant increased risk in hospitalizations and death from asthma problems.
- Advair HFA is not for adults and adolescents with asthma who are well controlled with an asthma control medicine, such as a low to medium dose of an ICS medicine. Advair HFA is for adults and adolescents with asthma who need both an ICS and LABA medicine.
Who should not use Advair HFA?
Do not use Advair HFA:
- to relieve sudden breathing problems.
- as a rescue inhaler.
- if you are allergic to fluticasone propionate, salmeterol, or any of the ingredients in Advair HFA. See the end of this Patient Information for a complete list of ingredients in Advair HFA.
What should I tell my healthcare provider before using Advair HFA?
Before using Advair HFA, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have high blood pressure.
- have seizures.
- have thyroid problems.
- have diabetes.
- have liver problems.
- have weak bones (osteoporosis).
- have an immune system problem.
- have or have had eye problems such as glaucoma, increased pressure in your eye, cataracts, or other changes in vision.
- have any type of viral, bacterial, or fungal infection.
- are exposed to chickenpox or measles.
- are pregnant or plan to become pregnant. It is not known if Advair HFA may harm your unborn baby.
- are breastfeeding. It is not known if the medicines in Advair HFA pass into your milk and if they can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Advair HFA and certain other medicines may interact with each other. This may cause serious side effects. Especially tell your healthcare provider if you take antifungal or anti-HIV medicines.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Advair HFA?
Read the step-by-step instructions for using Advair HFA that comes with the medication.
- Do not use Advair HFA unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- Advair HFA comes in 3 different strengths. Your healthcare provider prescribed the strength that is best for you.
- Use Advair HFA exactly as your healthcare provider tells you to use it. Do not use Advair HFA more often than prescribed.
- Use 2 inhalations of Advair HFA 2 times each day. Use Advair HFA at the same time each day, about 12 hours apart.
- If you miss a dose of Advair HFA, just skip that dose. Take your next dose at your usual time. Do not take 2 doses at 1 time.
- If you take too much Advair HFA, call your healthcare provider or go to the nearest hospital emergency room right away if you have any unusual symptoms, such as:
- worsening shortness of breath
- chest pain
- increased heart rate
- shakiness
- Do not use other medicines that contain a LABA for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA medicines.
- Do not stop using Advair HFA, even if you are feeling better, unless your healthcare provider tells you to.
- Advair HFA does not relieve sudden breathing problems. Always have a rescue inhaler with you to treat sudden symptoms. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- Rinse your mouth with water without swallowing after each dose of Advair HFA. This will help lessen the chance of getting a yeast infection (thrush) in your mouth and throat.
- Call your healthcare provider or get medical care right away if:
- your breathing problems get worse.
- you need to use your rescue inhaler more often than usual.
- your rescue inhaler does not work as well to relieve your symptoms.
- you need to use 4 or more inhalations of your rescue inhaler in 24 hours for 2 or more days in a row.
- you use 1 whole canister of your rescue inhaler in 8 weeks.
- your peak flow meter results decrease. Your healthcare provider will tell you the numbers that are right for you.
- you have asthma and your symptoms do not improve after using Advair HFA regularly for 1 week.
What are the possible side effects of Advair HFA?
Advair HFA can cause serious side effects, including:
- fungal infection in your mouth or throat (thrush). Rinse your mouth with water without swallowing after using Advair HFA to help reduce your chance of getting thrush.
- pneumonia. Advair HFA contains the same medicine found in Advair Diskus (fluticasone propionate and salmeterol inhalation powder). Advair Diskus is used to treat people with asthma and people with chronic obstructive pulmonary disease (COPD). People with COPD have a higher chance of getting pneumonia. Advair Diskus may increase the chance of you getting pneumonia. It is not known if Advair HFA is safe and effective in people with COPD. Call your healthcare provider right away if you have any of the following symptoms:
- increase in mucus (sputum) production
- chills
- change in mucus color
- increased cough
- fever
- increased breathing problems
- weakened immune system and increased chance of getting infections (immunosuppression).
- reduced adrenal function (adrenal insufficiency). Adrenal insufficiency is a condition where the adrenal glands do not make enough steroid hormones. This can happen when you stop taking oral corticosteroid medicines (such as prednisone) and start taking a medicine containing an inhaled steroid (such as Advair HFA). During this transition period, when your body is under stress such as from fever, trauma (such as a car accident), infection, surgery, or worse COPD symptoms, adrenal insufficiency can get worse and may cause death. Symptoms of adrenal insufficiency include:
- feeling tired
- nausea and vomiting
- lack of energy
- low blood pressure (hypotension)
- weakness
- sudden breathing problems immediately after inhaling your medicine. If you have sudden breathing problems immediately after inhaling your medicine, stop using Advair HFA and call your healthcare provider right away.
- serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction:
- rash
- swelling of your face, mouth, and tongue
- hives
- breathing problems
- effects on heart.
- increased blood pressure
- chest pain
- a fast or irregular heartbeat
- effects on nervous system.
- tremor
- nervousness
- bone thinning or weakness (osteoporosis).
- slowed growth in children. Your child’s growth should be checked regularly by the healthcare provider while using Advair HFA.
- eye problems including glaucoma, increased pressure in your eye, cataracts, or other changes in vision. You should have regular eye exams while using Advair HFA.
- changes in laboratory blood levels (sugar, potassium, certain types of white blood cells).
Common side effects of Advair HFA include:
- upper respiratory tract infection
- headache
- throat irritation
- dizziness
- hoarseness and voice changes
- nausea and vomiting
These are not all the possible side effects of Advair HFA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Advair HFA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Advair HFA for a condition for which it was not prescribed. Do not give Advair HFA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Advair HFA that was written for health professionals.
How should I store Advair HFA?
- Store Advair HFA at room temperature between 68°F and 77°F (20°C and 25°C) with the mouthpiece down.
- The contents of your Advair HFA are under pressure: Do not puncture. Do not use or store near heat or open flame. Temperatures above 120°F may cause the canister to burst.
- Do not throw into fire or an incinerator.
- Safely throw away Advair HFA in the trash when the counter reads 000.
Keep Advair HFA and all medicines out of the reach of children.
What are the ingredients in Advair HFA?
Active ingredients: fluticasone propionate, salmeterol xinafoate
Inactive ingredient: propellant HFA-134a
For more information about Advair HFA, call 1-888-825-5249.
Instructions for use for Advair HFA
Advair (AD vair) HFA
(fluticasone propionate and salmeterol inhalation aerosol)
for oral inhalation use
Your Advair HFA inhaler
- The metal canister holds the medicine. See Figure A.
- The metal canister has a counter to show how many sprays of medicine you have left. The number shows through a window in the back of the purple plastic actuator. See Figure A.
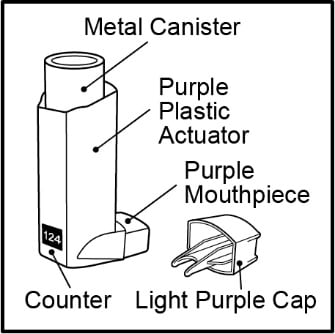
Figure A
- The counter starts at either 124 or 064, depending on which size inhaler you have. The number will count down by 1 each time you spray the inhaler. The counter will stop counting at 000.
- Do not try to change the numbers or take the counter off the metal canister. The counter cannot be reset, and it is permanently attached to the metal canister.
- The purple plastic actuator sprays the medicine from the metal canister. The plastic actuator has a light purple protective cap that covers the mouthpiece. See Figure A. Keep the protective cap on the mouthpiece when the metal canister is not in use.
- Do not use the plastic actuator with a canister of medicine from any other inhaler.
- Do not use an Advair HFA metal canister with an actuator from any other inhaler.
Before using your Advair HFA inhaler
- The inhaler should be at room temperature before you use it.
Priming your Advair HFA inhaler
Before you use Advair HFA for the first time, you must prime the inhaler so that you will get the right amount of medicine when you use it.
- To take the cap off the mouthpiece, squeeze the sides of the cap and pull it straight out. See Figure B.
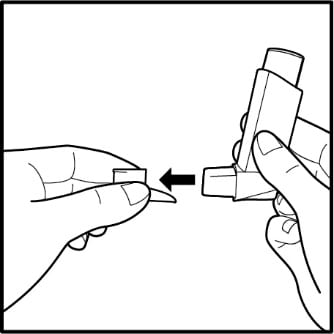
Figure B
- Shake the inhaler well for 5 seconds.
- Spray the inhaler 1 time into the air away from your face. Avoid spraying in eyes. See Figure C.
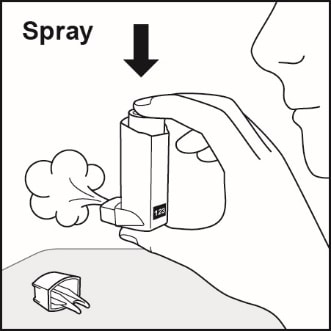
Figure C
- Shake and spray the inhaler like this 3 more times to finish priming it. The counter should now read 120 or 060, depending on which size inhaler you have. See Figure D.
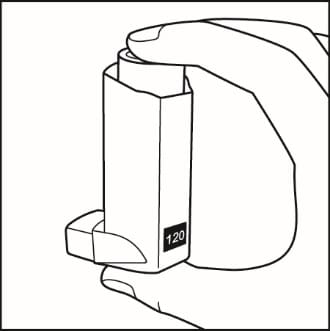
Figure D
- You must prime your inhaler again if you have not used it in more than 4 weeks or if you have dropped it. To take the cap off the mouthpiece, squeeze the sides of the cap and pull it straight out. Shake the inhaler well for 5 seconds. Then spray it 1 time into the air away from your face. Shake and spray the inhaler like this 1 more time to finish priming it.
How to use your Advair HFA inhaler
Follow these steps every time you use Advair HFA.
Step 1. Make sure the metal canister fits firmly in the plastic actuator. The counter should show through the window in the actuator.
To take the cap off the mouthpiece, squeeze the sides of the cap and pull it straight out.
Look inside the mouthpiece for foreign objects and take out any you see.
Step 2. Hold the inhaler with the mouthpiece down and shake it well for 5 seconds . See Figure E.
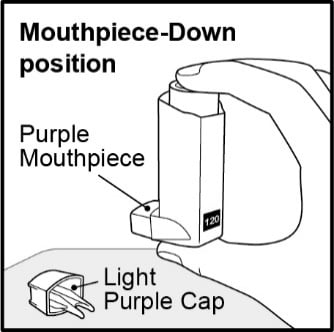
Figure E
Step 3. Breathe out through your mouth and push as much air from your lungs as you can. See Figure F.
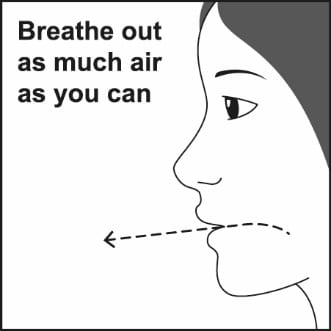
Figure F
Step 4. Put the mouthpiece in your mouth and close your lips around it. Push the top of the metal canister all the way down while you breathe in deeply and slowly through your mouth. See Figure G.
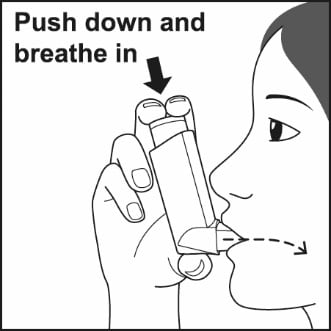
Figure G
Step 5. After the spray comes out, take your finger off the metal canister. After you have breathed in all the way, take the inhaler out of your mouth and close your mouth.
Step 6. Hold your breath for about 10 seconds, or for as long as is comfortable. Breathe out slowly as long as you can.
Wait about 30 seconds and shake the inhaler well for 5 seconds. Repeat Step 2 through Step 6.
Step 7. Rinse your mouth with water after breathing in the medicine. Spit out the water. Do not swallow it. See Figure H.
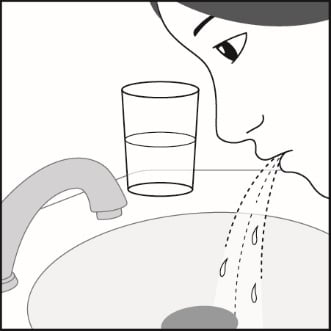
Figure H
Step 8. Put the cap back on the mouthpiece after every time you use the inhaler. Make sure it snaps firmly into place.
Cleaning your Advair HFA inhaler
Clean your inhaler at least 1 time each week after your evening dose. You may not see any medicine build-up on the inhaler, but it is important to keep it clean so medicine build-up will not block the spray. See Figure I.
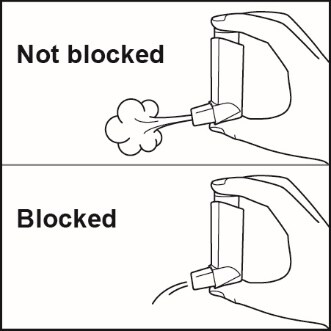
Figure I
Step 9.Take the cap off the mouthpiece by squeezing the sides of the cap and pulling it straight out. Do not take the metal canister out of the plastic actuator.
Step 10. Use a dry cotton swab to clean the small circular opening where the medicine sprays out of the canister. Carefully twist the swab in a circular motion to take off any medicine. See Figure J.
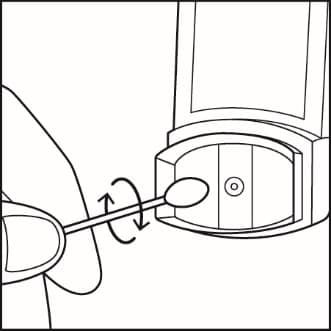
Figure J
Step 11. Wipe the inside of the mouthpiece with a clean tissue dampened with water. Let the plastic actuator air-dry overnight.
Step 12. Put the cap back on the mouthpiece after the plastic actuator has dried.
Replacing your Advair HFA inhaler
- When the counter reads 020, you should refill your prescription or ask your healthcare provider if you need another prescription for Advair HFA.
- When the counter reads 000, throw the inhaler away. You should not keep using the inhaler when the counter reads 000 because you may not receive the right amount of medicine.
- Do not use the inhaler after the expiration date, which is on the packaging it comes in.
For correct use of your Advair HFA inhaler, remember:
- The metal canister should always fit firmly in the plastic actuator.
- Breathe in deeply and slowly to make sure you get all the medicine.
- Hold your breath for about 10 seconds after breathing in the medicine. Then breathe out fully.
- After each dose, rinse your mouth with water and spit it out. Do not swallow the water.
- Do not take the inhaler apart.
- Always keep the protective cap on the mouthpiece when your inhaler is not in use.
- Always store your inhaler with the mouthpiece pointing down.
- Clean your inhaler at least 1 time each week.
For more information about Advair HFA or how to use your inhaler, call 1-888-825-5249.
Instructions for use revised 08/2021.