What is Hulio?
Hulio is a medicine called a Tumor Necrosis Factor (TNF) blocker. Hulio is used:
- To reduce the signs and symptoms of:
- moderate to severe rheumatoid arthritis (RA) in adults. Hulio can be used alone, with methotrexate, or with certain other medicines.
- moderate to severe polyarticular juvenile idiopathic arthritis (JIA) in children 2 years and older. Hulio can be used alone or with methotrexate.
- psoriatic arthritis (PsA) in adults. Hulio can be used alone or with certain other medicines.
- ankylosing spondylitis (AS) in adults.
- To treat moderate to severe Crohn’s disease (CD) in adults and children 6 years of age and older.
- To treat moderate to severe ulcerative colitis (UC) in adults. It is not known if adalimumab products are effective in people who stopped responding to or could not tolerate TNF-blocker medicines.
- To treat moderate to severe chronic (lasting a long time) plaque psoriasis (Ps) in adults who have the condition in many areas of their body and who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet light alone or with pills).
What is the most important information I should know about Hulio?
Hulio is a medicine that affects your immune system. Hulio can lower the ability of your immune system to fight infections. Serious infections have happened in people taking adalimumab products. These serious infections include tuberculosis (TB) and infections caused by viruses, fungi or bacteria that have spread throughout the body. Some people have died from these infections.
- Your healthcare provider should test you for TB before starting Hulio.
- Your healthcare provider should check you closely for signs and symptoms of TB during treatment with Hulio.
You should not start taking Hulio if you have any kind of infection unless your healthcare provider says it is okay.
Before starting Hulio, tell your healthcare provider if you:
- think you have an infection or have symptoms of an infection such as:
- fever, sweats, or chills
- warm, red, or painful skin or sores
- muscle aches on your body
- cough
- diarrhea or stomach pain
- shortness of breath
- burning when you urinate or urinate
- blood in phlegm more often than normal
- feel very tired
- weight loss
- are being treated for an infection
- get a lot of infections or have infections that keep coming back
- have diabetes
- have TB, or have been in close contact with someone with TB
- were born in, lived in, or traveled to countries where there is more risk for getting TB.
Ask your healthcare provider if you are not sure. - live or have lived in certain parts of the country (such as the Ohio and Mississippi River valleys) where there is an increased risk for getting certain kinds of fungal infections (histoplasmosis, coccidioidomycosis, or blastomycosis). These infections may happen or become more severe if you use Hulio. Ask your healthcare provider if you do not know if you have lived in an area where these infections are common.
- have or have had hepatitis B
- use the medicine Orencia (abatacept), Kineret (anakinra), Rituxan (rituximab), Imuran (azathioprine), or Purinethol (6–mercaptopurine, 6-MP).
- are scheduled to have major surgery
After starting Hulio, call your healthcare provider right away if you have an infection, or any sign of an infection.
Hulio can make you more likely to get infections or make any infection that you may have worse.
Cancer
- For children and adults taking Tumor Necrosis Factor (TNF)-blockers, including Hulio, the chances of getting cancer may increase.
- There have been cases of unusual cancers in children, teenagers, and young adults using TNF-blockers.
- People with RA, especially more serious RA, may have a higher chance for getting a kind of cancer called lymphoma.
- If you use TNF blockers including Hulio your chance of getting two types of skin cancer may increase (basal cell cancer and squamous cell cancer of the skin). These types of cancer are generally not life-threatening if treated. Tell your healthcare provider if you have a bump or open sore that does not heal.
- Some people receiving TNF blockers including Hulio developed a rare type of cancer called hepatosplenic T-cell lymphoma. This type of cancer often results in death. Most of these people were male teenagers or young men. Also, most people were being treated for Crohn’s disease or ulcerative colitis with another medicine called Imuran (azathioprine) or Purinethol (6-mercaptopurine, 6–MP).
What should I tell my healthcare provider before taking Hulio?
Hulio may not be right for you. Before starting Hulio, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection. See “What is the most important information I should know about Hulio?”
- have or have had cancer.
- have any numbness or tingling or have a disease that affects your nervous system such as multiple sclerosis or Guillain-Barré syndrome.
- have or had heart failure.
- have recently received or are scheduled to receive a vaccine. You may receive vaccines, except for live vaccines while using Hulio. Children should be brought up to date with all vaccines before starting Hulio.
- are allergic to Hulio or to any of its ingredients. See below for a list of ingredients in Hulio.
- are pregnant or plan to become pregnant, breastfeeding or plan to breastfeed. You and your healthcare provider should decide if you should take Hulio while you are pregnant or breastfeeding.
- have a baby and you were using Hulio during your pregnancy. Tell your baby’s healthcare provider before your baby receives any vaccines.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you use:
- Orencia (abatacept), Kineret (anakinra), Remicade (infliximab), Enbrel (etanercept), Cimzia (certolizumab pegol) or Simponi (golimumab), because you should not use Hulio while you are also using one of these medicines.
- Rituxan (rituximab). Your healthcare provider may not want to give you Hulio if you have received Rituxan (rituximab) recently.
- Imuran (azathioprine) or Purinethol (6–mercaptopurine, 6-MP).
Keep a list of your medicines with you to show your healthcare provider and pharmacist each time you get a new medicine.
How should I take Hulio?
- Hulio is given by an injection under the skin. Your healthcare provider will tell you how often to take an injection of Hulio. This is based on your condition to be treated. Do not inject Hulio more often than you were prescribed.
- See the Instructions for Use inside the carton for complete instructions for the right way to prepare and inject Hulio.
- Make sure you have been shown how to inject Hulio before you do it yourself. You can call your healthcare provider or 1-800-796-9526 if you have any questions about giving yourself an injection. Someone you know can also help you with your injection after they have been shown how to prepare and inject Hulio.
- Do not try to inject Hulio yourself until you have been shown the right way to give the injections. If your healthcare provider decides that you or a caregiver may be able to give your injections of Hulio at home, you should receive training on the right way to prepare and inject Hulio.
- Do not miss any doses of Hulio unless your healthcare provider says it is okay. If you forget to take Hulio, inject a dose as soon as you remember. Then, take your next dose at your regular scheduled time. This will put you back on schedule. In case you are not sure when to inject Hulio, call your healthcare provider or pharmacist.
- If you take more Hulio than you were told to take, call your healthcare provider.
What are the possible side effects of Hulio?
Hulio can cause serious side effects, including:
See “What is the most important information I should know about Hulio?”
- Serious Infections.
Your healthcare provider will examine you for TB and perform a test to see if you have TB. If your healthcare provider feels that you are at risk for TB, you may be treated with medicine for TB before you begin treatment with Hulio and during treatment with Hulio. Even if your TB test is negative your healthcare provider should carefully monitor you for TB infections while you are taking Hulio. People who had a negative TB skin test before receiving adalimumab products have developed active TB. Tell your healthcare provider if you have any of the following symptoms while taking or after taking Hulio:- cough that does not go away
- weight loss
- low grade fever
- loss of body fat and muscle (wasting)
- Hepatitis B infection in people who carry the virus in their blood.
If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus can become active while you use Hulio. Your healthcare provider should do blood tests before you start treatment, while you are using Hulio, and for several months after you stop treatment with Hulio. Tell your healthcare provider if you have any of the following symptoms of a possible hepatitis B infection:- muscle aches
- clay-colored bowel movements
- feel very tired
- fever
- dark urine
- chills
- skin or eyes look yellow
- stomach discomfort
- little or no appetite
- skin rash
- vomiting
- Allergic reactions. Allergic reactions can happen in people who use Hulio. Call your healthcare provider or get medical help right away if you have any of these symptoms of a serious allergic reaction:
- hives
- swelling of your face, eyes, lips or mouth
- trouble breathing
- Nervous system problems. Signs and symptoms of a nervous system problem include: numbness or tingling, problems with your vision, weakness in your arms or legs, and dizziness.
- Blood problems. Your body may not make enough of the blood cells that help fight infections or help to stop bleeding. Symptoms include a fever that does not go away, bruising or bleeding very easily, or looking very pale.
- New heart failure or worsening of heart failure you already have. Call your healthcare provider right away if you get new worsening symptoms of heart failure while taking Hulio, including:
- shortness of breath
- swelling of your ankles or feet
- sudden weight gain
- Immune reactions including a lupus-like syndrome. Symptoms include chest discomfort or pain that does not go away, shortness of breath, joint pain, or a rash on your cheeks or arms that gets worse in the sun. Symptoms may improve when you stop Hulio.
- Liver problems. Liver problems can happen in people who use TNF-blocker medicines. These problems can lead to liver failure and death. Call your healthcare provider right away if you have any of these symptoms:
- feel very tired
- skin or eyes look yellow
- poor appetite or vomiting
- pain on the right side of your stomach (abdomen)
- Psoriasis. Some people using adalimumab products had new psoriasis or worsening of psoriasis they already had. Tell your healthcare provider if you develop red scaly patches or raised bumps that are filled with pus. Your healthcare provider may decide to stop your treatment with Hulio.
Call your healthcare provider or get medical care right away if you develop any of the above symptoms. Your treatment with Hulio may be stopped.
The most common side effects of Hulio include:
- injection site reactions: redness, rash, swelling, itching, or bruising. These symptoms usually will go away within a few days. Call your healthcare provider right away if you have pain, redness or swelling around the injection site that does not go away within a few days or gets worse.
- upper respiratory infections (including sinus infections).
- headaches.
- rash.
These are not all the possible side effects with Hulio. Tell your healthcare provider if you have any side effect that bothers you or that does not go away. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Hulio
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Hulio for a condition for which it was not prescribed. Do not give Hulio to other people, even if they have the same condition. It may harm them. This Medication Guide summarizes the most important information about Hulio. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Hulio that is written for health professionals.
How should I store Hulio?
- Store Hulio in the refrigerator at 36ºF to 46ºF (2ºC to 8ºC). Store Hulio in the original carton until use to protect it from light.
- Do not freeze Hulio. Do not use Hulio if frozen, even if it has been thawed.
- Refrigerated Hulio may be used until the expiration date printed on the Hulio carton, dose tray, Pen or prefilled syringe. Do not use Hulio after the expiration date.
- If needed, for example when you are traveling, you may also store Hulio at room temperature up to 77°F (25°C) for up to 14 days. Store Hulio in the original carton until use to protect it from light.
- Throw away Hulio if it has been kept at room temperature and not been used within 14 days.
- Record the date you first remove Hulio from the refrigerator in the spaces provided on the carton and dose tray.
- Do not store Hulio in extreme heat or cold.
- Do not use a Pen or prefilled syringe if the liquid is cloudy, discolored, or has flakes or particles in it.
Keep Hulio, injection supplies, and all other medicines out of the reach of children.
What are the ingredients in Hulio?
Active ingredient: adalimumab-fkjp
Hulio Pen 40 mg/0.8 mL, Hulio 40 mg/0.8 mL prefilled syringe, Hulio 20 mg/0.4 mL prefilled syringe.
Inactive ingredients: methionine, monosodium glutamate, polysorbate 80, sorbitol and Water for Injection, USP. Hydrochloric acid is added as necessary to adjust pH.
For more information, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or you can enroll in a patient support program by calling 1-800-796-9526.
Instructions for use for Hulio
Hulio Pen (hue 'lee oh)
(adalimumab-fkjp) injection
for subcutaneous use
40 mg/0.8 mL
Single-Dose Prefilled Pen
For subcutaneous (under the skin) use only
Read these instructions carefully before using your Pen. This information does not replace talking to your healthcare provider about your medical condition and your treatment.
Do not try to inject Hulio yourself until you have been shown the right way to give the injections and have read and understand this Instructions for Use. If your healthcare provider decides that you or a caregiver may be able to give your injections of Hulio at home, you should receive training on the right way to prepare and inject Hulio. It is important that you read, understand, and follow these instructions so that you inject Hulio the right way. It is also important to talk to your healthcare provider to be sure you understand your Hulio dosing instructions. To help you remember when to inject Hulio, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver has any questions about the right way to inject Hulio.
For Questions or assistance, Call Mylan at 1-877-446-3679 (1-877-4-INFO-RX)
|
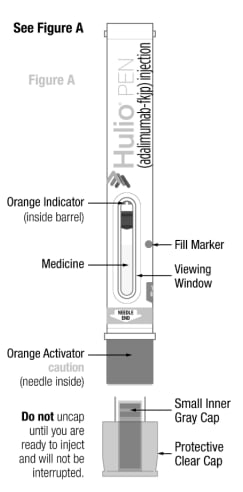
Caution: Never put your thumb, fingers, or hand over the orange activator after cap is removed. Never press or push the orange activator with your thumb, fingers, or hand. The orange activator is where the needle comes out. If accidental injection to your fingers or hands occurs, apply first-aid and either call your healthcare provider or go to the nearest hospital emergency room if needed. |
Dosage: Hulio Pen is for single dose (1-time) use only.
Important:
- Do not use Hulio if frozen, even if it has been thawed.
- Do not uncap your Hulio Pen until you are ready to inject and will not be interrupted.
- Do not recap. Recapping your Hulio Pen can damage the needle.
A Ioud “click” will occur when the orange activator is pressed down to deliver your dose of Hulio.
- You must practice injecting with your healthcare provider or nurse so that you are not surprised by this “click” sound when you give yourself injections.
- This 1st “click” sound means the start of the injection.
- You wiII know that the injection has finished when aII three of these have occurred:
- A 2nd “click” was heard, and
- Orange Indicator has stopped and completely blocked the viewing window, and
- 10 seconds has passed.
Parts of the Hulio Pen
Storing and handling the Hulio Pen
- Store Pen in a refrigerator between 36°F to 46°F (2°C to 8°C) in its original carton.
- Refrigerated Hulio may be used until the expiration date.
- Do not freeze the Pen.
- Do not expose the Pen to extreme heat or cold, such as a hot car, or a freezing car overnight.
If needed, for example when traveling, Hulio may be stored at room temperature up to 77°F (25°C) for a period of up to 14 days, with protection from light. Discard (throw away) Hulio if not used within the 14 day period. Record the date on the carton and dose tray when Hulio is first removed from the refrigerator.
- Protect from light.
- Take care not to drop or crush.
- Do not use if the Pen is damaged or broken.
- Keep Hulio, injection supplies, and all other medicines out of the reach of children.
Gather supplies for injection
Find a quiet area with a well-lit, clean and flat work surface and gather all the supplies you will need to give yourself or receive an injection.
Supplies you will need:
Included in Hulio carton
- 1 Pen (taken from refrigerator 30 minutes prior to intended injection time to allow Pen to reach room temperature.)
- 1 alcohol prep
Not included in Hulio carton
- 1 FDA-cleared sharps disposal container or puncture resistant container. (See “How should I throw away (dispose of) the used Hulio Pen and Cap?” Section in Step 7 at the end of this Instructions for Use).
- 1 gauze pad or cotton ball
If you do not have all the supplies you need to give yourself an injection, visit or call your local pharmacist.
Preparing the Pen
Remove the Pen from the refrigerator 30 minutes before using.
- Make sure the name Hulio appears on the Pen label.
- Check the expiration date printed on the Pen (See Figure B).
- Do not use the Pen past the expiration date.
- Check that the Hulio Pen has not been frozen or left in direct sunlight.
- Let the Pen reach room temperature.
- Do not use external heat sources such as hot water, direct sunlight, or microwave to warm the Pen.
- Do not put the Pen back in refrigerator after it has reached room temperature.

Figure B
Check the viewing window to make sure:
- Medicine is at or near the fill marker. You may need to shake gently to see liquid.
- Medicine is clear and colorless to pale brownish-yellow (See Figure C).
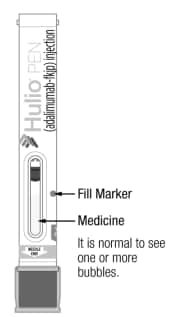
Figure C
Do not use the Pen if medicine is not near the fill marker. Use another Pen or contact your healthcare provider.
Do not use the Pen if it is cloudy, discolored, or has particles in it.
Choosing and preparing injection site
Your healthcare provider should show you proper injection site techniques.
- Recommended subcutaneous (under the skin) injection sites are:
- the front of the thighs, or
- the abdomen (belly)
- Do not use area within 2 inches of belly button (See Figure D).
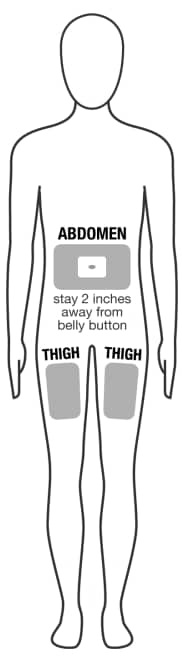
Figure D
- You should rotate and change your injection site each time you give an injection.
- Stay at least 1 inch from a previous site used.
- Do not inject into areas where the skin is tender, bruised, red, hard, scarred or has stretch marks.
- If you have psoriasis, do not inject into any raised, thick, red, or scaly skin patches or lesions.
- Do not inject through clothes. Roll back any clothing that may get in the way of the injection site.
- Wash your hands with soap and water.
- Wipe the chosen injection site using a circular motion with an alcohol prep. Wait for it to dry on its own, do not blow dry.
- Do not touch this injection site again before receiving your injection.
Giving the injection
Caution: Injection process must be completed without interruption. Read all steps first before beginning injection.
Step 1. Uncap
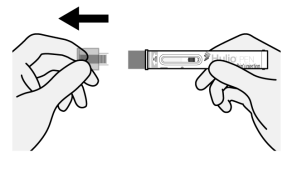
Figure E
- Pull straight on the protective clear cap to uncap the Pen (See Figure E). Do not twist.
- A few drops of liquid may come out of the needle, this is normal.
- Throw away the protective clear cap in an FDA-cleared sharps container or puncture resistant container (See “How should I throw away (dispose of) the used Hulio Pen and Cap?” in Step 7). Do not put your protective clear cap in the household trash.
Important:
- Do not put the protective clear cap back on the Pen. This can damage the needle.
- Do not touch the orange activator with your fingers (this is where the needle comes out).
Step 2. Squeeze and hold injection site
The thigh injection site is shown here (See Figure F). Perform these steps the same way for abdomen (belly) injection sites.
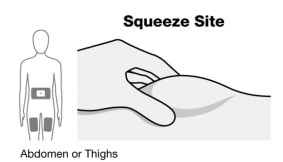
Figure F
- Squeeze the injection site to create a raised area, and hold that area firmly, until the injection is complete.
- See “Choosing and preparing injection site” or talk to your healthcare provider for injection site assistance.
Step 3. Place Pen
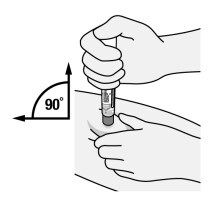
Figure G
- Place the orange activator end onto the injection site.
- Keep the Pen held straight (90° angle) and flat to the raised injection site area, and with the viewing window visible to you (See Figure G).
- Be careful to place the Pen so that it will not inject into your fingers holding the injection site.
Step 4. Begin injection
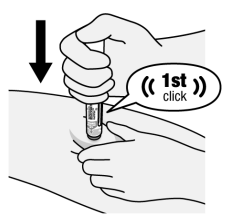
Figure H
- Firmly push the body of the Pen down against the injection site to engage the orange activator and begin injection (See Figure H). Try not to cover the viewing window.
- Continue pushing down after hearing the 1st “click”. This 1st “click” sound signals the start of the injection.
- In the viewing window, the Orange-Indicator will move to show the progress of the injection.
- Do not move, twist, or rotate the Pen during injection.
Step 5. click, 10 seconds, orange indicator
Hold down for 2nd “click”, orange indicator and 10 seconds
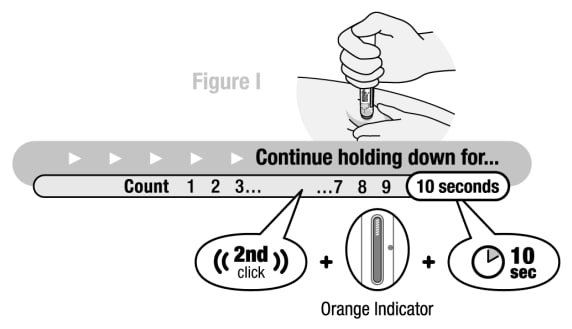
Figure I
Continue pushing the body of the Pen down against the injection site until:
- A 2nd “click” was heard, and
- Orange indicator has stopped and completely blocked the viewing window (See Figure I), and
- 10 seconds has passed.
Caution: Make sure all three of these have occurred to ensure all medicine was delivered. If the needle did not retract or you do not think you received the full dose contact your healthcare provider for assistance.
Step 6. End of injection, remove Hulio Pen
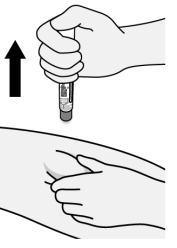
Figure J
- Pull Pen straight away from injection site (See Figure J).
- There may be a small amount of liquid on the injection site. This is normal. If slight bleeding occurs from the injection site, press a gauze pad or cotton ball lightly against the site for a few seconds.
- Do not rub the injection site.
Dispose of the Hulio Pen and Cap
Put the used Pen and Cap in an FDA-cleared sharps disposal container or puncture resistant container right away to avoid injury (See “How should I throw away (dispose of) the used Hulio Pen and Cap?” in Step 7).
Pen is for single-dose only.
- Do not reuse the Pen if all of the medicine was not injected.
- Do not try to recap the Pen as it could lead to a needle stick injury.
Step 7. How should I throw away (dispose of) the used Hulio Pen and Cap?
- Put your used Pen and cap in an FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) the Pen or Cap in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and Pens. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
Do not recycle your used sharps disposal container.
Step 8. Write down the date you received your injection and the injection site used in the injection diary.
Customer Service For Questions or Assistance, Call Mylan at 1-877-446-3679 (1-877-4-INFO-RX)
Instructions for use approved 07/2022




