Boxed Warning
Hypersensitivity reactions:
Serious and sometimes fatal hypersensitivity reactions, with multiple organ involvement, have occurred with abacavir. Patients who carry the HLA-B*5701 allele are at a higher risk of a hypersensitivity reaction to abacavir; although, hypersensitivity reactions have occurred in patients who do not carry the HLA-B*5701 allele.
Abacavir/lamivudine/zidovudine is contraindicated in patients with a prior hypersensitivity reaction to abacavir and in HLA-B*5701-positive patients. All patients should be screened for the HLA-B*5701 allele prior to initiating therapy with abacavir/lamivudine/zidovudine or reinitiation of therapy with abacavir/lamivudine/zidovudine, unless patients have a previously documented HLA-B*5701 allele assessment. Discontinue abacavir/lamivudine/zidovudine immediately if a hypersensitivity reaction is suspected, regardless of HLA-B*5701 status and even when other diagnoses are possible.
Following a hypersensitivity reaction to abacavir, never restart abacavir/lamivudine/zidovudine or any other abacavir-containing product because more severe symptoms, including death, can occur within hours. Similar severe reactions have also occurred rarely following the reintroduction of abacavir-containing products in patient who have no history of abacavir hypersensitivity.
Hematologic toxicity:
Zidovudine has been associated with hematologic toxicity, including neutropenia and severe anemia, particularly in patients with advanced HIV-1 disease.
Myopathy:
Prolonged use of zidovudine has been associated with symptomatic myopathy.
Lactic acidosis and severe hepatomegaly with steatosis:
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues. Discontinue abacavir/lamivudine/zidovudine if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Exacerbations of hepatitis B:
Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with hepatitis B virus (HBV) and HIV-1 and have discontinued lamivudine, which is one component of abacavir/lamivudine/zidovudine. Monitor hepatic function closely with both clinical and laboratory follow-up for at least several months in patients who discontinue abacavir/lamivudine/zidovudine and are coinfected with HIV-1 and HBV. If appropriate, initiation of anti-HBV therapy may be warranted.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
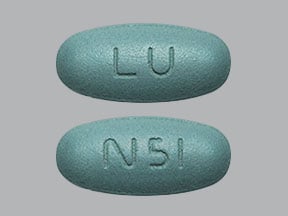
Trizivir: Abacavir sulfate 300 mg, lamivudine 150 mg, and zidovudine 300 mg [contains fd&c blue #2 (indigotine)]
Generic: Abacavir sulfate 300 mg, lamivudine 150 mg, and zidovudine 300 mg
Pharmacology
Mechanism of Action
The combination of abacavir, lamivudine, and zidovudine is believed to act synergistically to inhibit reverse transcriptase via DNA chain termination after incorporation of the nucleoside analogue as well as to delay the emergence of mutations conferring resistance.
Use: Labeled Indications
HIV infection: Treatment of HIV-1 infection in combination with other antiretroviral agents.
Contraindications
Hypersensitivity to abacavir, lamivudine, zidovudine, or any component of the formulation; patients positive for HLA-B*5701 allele; moderate or severe hepatic impairment.
Canadian labeling: Additional contraindications (not in US labeling): Hepatic impairment (regardless of severity of impairment); ANC <750 cells/mm3; hemoglobin <7.5 g/dL or 4.65 mmol/L
Dosage and Administration
Dosing: Adult
HIV-1 infection, treatment: Oral: One tablet twice daily.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
HIV-1 infection, treatment: May use alone or in combination with other antiretroviral agents:
Children and Adolescents <40 kg: Not recommended; product is a fixed-dose combination
Children and Adolescents ≥40 kg: Oral: 1 tablet twice daily
Administration
Administer without regard to food.
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Abacavir, Lamivudine, and Zidovudine Images
Drug Interactions
Acemetacin: May enhance the adverse/toxic effect of Zidovudine. Specifically, the risk for hematologic toxicity may be increased. Monitor therapy
Acyclovir-Valacyclovir: May enhance the CNS depressant effect of Zidovudine. Monitor therapy
Amodiaquine: Zidovudine may enhance the neutropenic effect of Amodiaquine. Avoid combination
BCG (Intravesical): Myelosuppressive Agents may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Cabozantinib: MRP2 Inhibitors may increase the serum concentration of Cabozantinib. Monitor therapy
Chloramphenicol (Ophthalmic): May enhance the adverse/toxic effect of Myelosuppressive Agents. Monitor therapy
Cladribine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Avoid combination
Cladribine: Agents that Undergo Intracellular Phosphorylation may diminish the therapeutic effect of Cladribine. Avoid combination
Clarithromycin: May enhance the myelosuppressive effect of Zidovudine. Clarithromycin may decrease the serum concentration of Zidovudine. Management: Monitor response to zidovudine closely when used with clarithromycin, and consider staggering zidovudine and clarithromycin doses when possible in order to minimize the potential for interaction. Consider therapy modification
CloZAPine: Myelosuppressive Agents may enhance the adverse/toxic effect of CloZAPine. Specifically, the risk for neutropenia may be increased. Monitor therapy
Deferiprone: Myelosuppressive Agents may enhance the neutropenic effect of Deferiprone. Management: Avoid the concomitant use of deferiprone and myelosuppressive agents whenever possible. If this combination cannot be avoided, monitor the absolute neutrophil count more closely. Consider therapy modification
Dexketoprofen: May enhance the adverse/toxic effect of Zidovudine. Monitor therapy
Dipyrone: May enhance the adverse/toxic effect of Myelosuppressive Agents. Specifically, the risk for agranulocytosis and pancytopenia may be increased Avoid combination
DOXOrubicin (Conventional): May enhance the adverse/toxic effect of Zidovudine. DOXOrubicin (Conventional) may diminish the therapeutic effect of Zidovudine. Consider therapy modification
DOXOrubicin (Liposomal): May enhance the adverse/toxic effect of Zidovudine. DOXOrubicin (Liposomal) may diminish the therapeutic effect of Zidovudine. Consider therapy modification
Emtricitabine: LamiVUDine may enhance the adverse/toxic effect of Emtricitabine. Avoid combination
Fluconazole: May decrease the metabolism of Zidovudine. Monitor therapy
Ganciclovir-Valganciclovir: May enhance the adverse/toxic effect of Zidovudine. Specifically, hematologic toxicity may be enhanced. Monitor therapy
Interferons: May enhance the adverse/toxic effect of Zidovudine. Interferons may decrease the metabolism of Zidovudine. Monitor therapy
Levomethadone: May increase the serum concentration of Zidovudine. Monitor therapy
Mesalamine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Methadone: May increase the serum concentration of Zidovudine. Monitor therapy
Methadone: May diminish the therapeutic effect of Abacavir. Abacavir may decrease the serum concentration of Methadone. Monitor therapy
Nitisinone: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Orlistat: May decrease the serum concentration of Antiretroviral Agents. Monitor therapy
Pretomanid: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Probenecid: May decrease the metabolism of Zidovudine. Monitor therapy
Promazine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Protease Inhibitors: May decrease the serum concentration of Zidovudine. Monitor therapy
Protease Inhibitors: May decrease the serum concentration of Abacavir. Monitor therapy
Raltegravir: May enhance the myopathic (rhabdomyolysis) effect of Zidovudine. Monitor therapy
Ribavirin (Oral Inhalation): Zidovudine may enhance the adverse/toxic effect of Ribavirin (Oral Inhalation). Specifically, the risk/severity of anemia may be increased. Management: Due to significantly increased risk of anemia, consider even closer monitoring for anemia than routinely recommended. Alternative therapies should be considered when clinically possible, particularly for patients with other risk factors. Consider therapy modification
Ribavirin (Systemic): Zidovudine may enhance the adverse/toxic effect of Ribavirin (Systemic). Specifically, the risk/severity of anemia may be increased. Management: Due to significantly increased risk of anemia, consider even closer monitoring for anemia than routinely recommended for ribavirin. Alternative therapies should be considered when clinically possible, particularly for patients with other risk factors. Consider therapy modification
Rifamycin Derivatives: May decrease the serum concentration of Zidovudine. Exceptions: Rifabutin. Monitor therapy
Sorbitol: May decrease the serum concentration of LamiVUDine. Management: When possible, avoid chronic coadministration of sorbitol-containing solutions with lamivudine, but if this combination cannot be avoided, monitor patients more closely for possible therapeutic failure associated with decreased lamivudine exposure. Consider therapy modification
Stavudine: Zidovudine may diminish the therapeutic effect of Stavudine. Avoid combination
Tenoxicam: May enhance the adverse/toxic effect of Zidovudine. Monitor therapy
Teriflunomide: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Tolvaptan: May increase the serum concentration of OAT1/3 Substrates. Management: Patients being treated with the Jynarque brand of tolvaptan should avoid concomitant use of OAT1/3 substrates. Concentrations and effects of the OAT1/3 substrate would be expected to increase with any combined use. Consider therapy modification
Trimethoprim: May increase the serum concentration of LamiVUDine. Monitor therapy
Valproate Products: May increase the serum concentration of Zidovudine. Monitor therapy
Adverse Reactions
See individual agents as well as other combination products for additional information. Frequency not always defined.
Central nervous system: Headache (13%), fatigue (12%), malaise (12%), depression (6%), anxiety (5%)
Dermatologic: Skin rash (5%)
Endocrine & metabolic: Increased amylase (2%), increased serum triglycerides (grade 3-4: 2%), increased gamma-glutamyl transferase, redistribution of body fat
Gastrointestinal: Nausea (19%), nausea and vomiting (10%), diarrhea (7%), pancreatitis
Hematologic & oncologic: Neutropenia (5%)
Hepatic: Increased serum ALT (6%)
Hypersensitivity: Hypersensitivity (1% to 9%; based on abacavir component; higher risk in carriers of the HLA-B*5701 allele)
Immunologic: Immune reconstitution syndrome
Infection: Viral infection (5%)
Miscellaneous: Fever and chills (6%)
Neuromuscular & skeletal: Increased creatine phosphokinase (7%)
Respiratory: ENT infection (5%)
<1%, postmarketing, and/or case reports: Abdominal pain, abnormal breath sounds, allergic sensitization (including anaphylaxis), alopecia, anemia, anorexia, aplastic anemia, arthralgia, cardiomyopathy, decreased appetite, dizziness, dyspepsia, erythema multiforme, exacerbation of hepatitis B (posttreatment), gynecomastia, increased serum bilirubin, increased serum transaminases, insomnia, lactic acidosis, liver steatosis, lymphadenopathy, myalgia, myasthenia, oral mucosa hyperpigmentation, paresthesia, peripheral neuropathy, rhabdomyolysis, seizure, sleep disorder, splenomegaly, Stevens-Johnson syndrome, stomatitis, thrombocytopenia, urticaria, vasculitis, weakness, wheezing
Warnings/Precautions
Concerns related to adverse effects:
- Hematologic toxicity: [US Boxed Warning]: Zidovudine has been associated with hematologic toxicities (eg, neutropenia, anemia); use with caution in patients with bone marrow compromise (eg, granulocyte count <1,000 cells/mm3 or hemoglobin <9.5 g/dL). Frequent complete blood counts are recommended in patients with advanced HIV-1 disease. Dosage interruption may be needed if anemia or neutropenia develops.
- Hypersensitivity reactions: [US Boxed Warning]: Serious hypersensitivity reactions (sometimes fatal) have occurred in patients taking abacavir (in Trizivir). Patients who carry the HLA-B*5701 allele are at a higher risk for a hypersensitivity reaction to abacavir, although hypersensitivity reactions have occurred in patients who do not carry the HLA-B*5701 allele. All patients should be screened for the HLA-B*5701 allele prior to initiating therapy with Trizivir or reinitiation of therapy with Trizivir unless patients have had a previously documented HLA-B*5701 allele assessment. Discontinue Trizivir if a hypersensitivity reaction is suspected. Trizivir is contraindicated in patients who have the HLA-B*5701 allele or in patients with a prior hypersensitivity reaction to abacavir. Reintroduction of Trizivir or any other abacavir-containing product can result in life-threatening or fatal hypersensitivity reactions, even in patients who have no history of hypersensitivity to abacavir therapy. Such reactions can occur within hours. Additionally, allele-positive patients (including abacavir treatment naive) should have an allergy to abacavir documented in their medical record. Reactions usually occur within 9 days of starting abacavir; ~90% occur within 6 weeks, although these reactions may occur at any time during therapy (HHS [adult] 2017). These reactions usually include signs or symptoms in 2 or more of the following groups: fever; rash; gastrointestinal (eg, nausea, vomiting, diarrhea, abdominal pain); constitutional (eg, generalized malaise, fatigue, achiness); respiratory (eg, dyspnea, cough, pharyngitis). Other signs and symptoms include lethargy, headache, myalgia, edema, abnormal chest x-ray findings, arthralgia and paresthesia. Anaphylaxis, liver failure, renal failure, hypotension, adult respiratory distress syndrome, respiratory failure, myolysis, and death have occurred in association with hypersensitivity reactions. Physical findings (lymphadenopathy, mucous membrane lesions, and rash [maculopapular, urticarial or variable]) may occur. Erythema multiforme has also been reported. Laboratory abnormalities (eg, elevated liver function tests, elevated creatine phosphokinase, elevated creatinine, and lymphopenia) may occur. Trizivir should be permanently discontinued if hypersensitivity cannot be ruled out, even when other diagnoses are possible. Following a hypersensitivity reaction, Trizivir SHOULD NOT be restarted because more severe symptoms may occur within hours, including LIFE-THREATENING HYPOTENSION AND DEATH. If Trizivir is to be restarted following an interruption in therapy not associated with symptoms of a hypersensitivity reaction, carefully evaluate the patient for previously unsuspected symptoms of hypersensitivity. Do not restart if hypersensitivity is suspected or cannot be ruled out regardless of HLA-B*5701 status. If Trizivir is restarted, continually monitor for symptoms of a hypersensitivity reaction. Make the patient aware that reintroduction should only take place if medical care is readily accessible.
- Immune reconstitution syndrome: Patients may develop immune reconstitution syndrome resulting in the occurrence of an inflammatory response to an indolent or residual opportunistic infection during initial HIV treatment or activation of autoimmune disorders (eg, Graves disease, polymyositis, Guillain-Barré syndrome) later in therapy; further evaluation and treatment may be required.
- Lactic acidosis/hepatomegaly: [US Boxed Warning]: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues. Female gender and obesity may increase the risk for development. Suspend treatment in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or hepatotoxicity (transaminase elevation may/may not accompany hepatomegaly and steatosis).
- Lipoatrophy: May cause loss of subcutaneous fat, especially in the face, limbs, and buttocks. Lipoatrophy incidence and severity are related to cumulative exposure and may be only partially reversible; improvement may take months to years after switching to a regimen that does not contain zidovudine. Monitor patients for signs of lipoatrophy and consider switching to a non-zidovudine-containing regimen if lipoatrophy occurs.
- Myopathy: [US Boxed Warning]: Prolonged use of zidovudine has been associated with symptomatic myopathy and myositis.
- Pancreatitis: Pancreatitis has been observed with abacavir, lamivudine and zidovudine; rule out pancreatitis in patients who develop signs/symptoms (eg, nausea/vomiting, abdominal pain, elevated lipase, and amylase) during therapy.
Disease-related concerns:
- Chronic hepatitis B: [US Boxed Warning]: Exacerbation of hepatitis B (including fatalities) has been reported with discontinuation of lamivudine in coinfected HIV/HBV patients; monitor hepatic function (eg, serum ALT) and HBV viral DNA closely for several months after discontinuing Trizivir in coinfected patients.
- Coronary heart disease: Abacavir use has been associated with an increased risk of MI in some cohort studies (Elion 2018; HHS [adult] 2017). Consider using with caution in patients with risks for coronary heart disease and minimizing modifiable risk factors (eg, hypertension, hyperlipidemia, diabetes mellitus, and smoking) prior to use.
- Lamivudine-resistant HBV: Emergence of HBV virus variants associated with resistance to lamivudine have been reported in HIV-1 infected subjects who have received lamivudine-containing antiretroviral regimens in the presence of HBV coinfection.
- Renal impairment: Trizivir, as a fixed-dose combination tablet, should not be used in patients with CrCl <50 mL/minute.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pediatric patients <40 kg: Trizivir, as a fixed-dose combination tablet, should not be used in patients <40 kg or those requiring dosage adjustment.
- Therapy-experienced patients: Patients with prolonged prior nucleoside reverse transcriptase inhibitor (NRTI) exposure or presence of HIV-1 isolates containing multiple mutations conferring resistance to NRTIs have limited response to abacavir. The potential for cross resistance between abacavir and other NRTIs should be considered when evaluating new regimens in therapy experienced patients.
Monitoring Parameters
Blood glucose, CBC with differential, serum creatine kinase, CD4 count, HIV RNA plasma levels, bilirubin, serum transaminases, triglycerides, serum amylase; HLA-B*5701 genotype status prior to initiation of therapy and prior to reinitiation of therapy in patients of unknown HLA-B*5701 status; signs and symptoms of hypersensitivity, particularly in patients untested for the HLA-B*5701 allele; signs and symptoms of pancreatitis; observe for appearance of opportunistic infections
Pregnancy
Pregnancy Considerations
The Health and Human Services (HHS) perinatal HIV guidelines do not recommend use of this fixed-dose combination regimen in pregnancy. Women who become pregnant while taking this combination should be changed to a recommended regimen (HHS [perinatal] 2019).
Refer to individual monographs for additional information.
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience headache, anxiety, nausea, vomiting, or diarrhea. Have patient report immediately to prescriber signs of allergic reaction with organ failure (fever, rash, fatigue, flu-like signs, nausea, vomiting, diarrhea, abdominal pain, sore throat, cough, or difficulty breathing), signs of bone marrow depression (neutropenia or anemia), signs of liver problems (dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin), signs of lactic acidosis (fast breathing, fast heartbeat, abnormal heartbeat, vomiting, fatigue, shortness of breath, severe loss of strength and energy, severe dizziness, feeling cold, or muscle pain or cramps), signs of pancreatitis (severe abdominal pain, severe back pain, severe nausea, or vomiting), signs of kidney problems (unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain), severe loss of strength and energy, chest pain, depression, severe dizziness, passing out, mouth sores, muscle pain, muscle weakness, joint pain, burning or numbness feeling, shortness of breath, edema, change in body fat, or signs of infection (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.