What is Adlarity?
• Adlarity is a prescription medicine used to treat mild, moderate, and severe dementia of the Alzheimer’s type.
• It is not known if Adlarity is safe and effective in children.
Who should not use Adlarity?
Do not use Adlarity if you:
- are allergic to donepezil or to medicines that contain piperidines. See the end of this Patient Information leaflet for a complete list of ingredients in Adlarity.
- have had a skin reaction called allergic contact dermatitis to Adlarity
Ask your healthcare provider if you are not sure if you should use the Adlarity transdermal system.
What should I tell my healthcare provider before using Adlarity?
Before using Adlarity, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including problems with irregular, slow, or fast heartbeats.
- have stomach ulcers.
- have problems passing urine.
- have seizures.
- have asthma or other lung problems.
- are pregnant or plan to become pregnant. It is not known if Adlarity will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Adlarity passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Adlarity may affect the way other medicines work and other medicines may affect how Adlarity works.
Especially tell your healthcare provider if you take medicines called nonsteroidal anti-inflammatory drugs (NSAIDs). Ask your healthcare provider if you are not sure if any of your medicines are NSAIDs. Taking NSAIDs and Adlarity together may make you more likely to get stomach ulcers.
Adlarity taken with certain medicines used for anesthesia may cause side effects. Tell your healthcare provider or dentist that you use the Adlarity transdermal system before you have:
- surgery
- medical procedures
- dental surgery or procedures
Know the medicines that you take. Keep a list of all of your medicines. Show it to your healthcare provider before you start a new medicine.
How should I use Adlarity?
See the Instructions for Use at the end of this Patient Information leaflet for step-by-step instructions on how to apply, remove, and throw away (dispose of) Adlarity.
- Use Adlarity exactly how your healthcare provider tells you to use it.
- Adlarity is for skin use only.
- Apply 1 Adlarity transdermal system at a time to your skin 1 time weekly (every 7 days).
- Apply to clean, dry, intact skin with little to no hair.
- If your Adlarity transdermal system falls off, or if you miss a dose of Adlarity, apply a new transdermal system right away. You should remove your transdermal system 7 days later.
- If you stop using Adlarity, call your healthcare provider for instructions before you start using Adlarity again.
- In case of overdose, get medical help or contact a live poison help line right away.
What should I avoid while using Adlarity?
- Do not touch your eyes after you touch the Adlarity transdermal system. In case of accidental contact with your eyes, or if your eyes become red after handling the transdermal system, rinse your eyes right away with water and get medical help if symptoms do not go away.
- Avoid exposure to heat sources, such as excessive sunlight, saunas, sunrooms, or heating pads, for long periods of time. Too much medicine could be absorbed into your body.
What are the possible side effects of Adlarity?
Adlarity may cause serious side effects, including:
- Skin reactions. Skin reactions that include redness and itching may happen at the application site when using Adlarity. Stop using Adlarity and call your healthcare provider if you get any of these skin reactions and they do not get better within 2 days (48 hours) after the transdermal system is removed:
- increased redness or swelling
- peeling or blistering of the skin
- spreading beyond the application site
- Slow heartbeat and fainting. Call your healthcare provider right away if you feel faint or lightheaded while using Adlarity.
- More stomach acid. This increases the chance of ulcers and bleeding. The risk is higher for some people, such as those who have had ulcers or take NSAIDs. Call your healthcare provider right away if you have any of these symptoms:
- heartburn or stomach pain that is new or does not go away.
- nausea or vomiting, blood in your vomit, or dark vomit that looks like coffee grounds.
- bowel movements or stools that look like black tar.
- Problems passing urine. Call your healthcare provider right away if you have problems passing urine while using Adlarity.
- Seizures. Call your healthcare provider right away if you have seizures while using Adlarity.
- Worsening of lung problems in people with asthma or other lung disease. Call your healthcare provider if you have new or worsening lung problems.
The most common side effects of donepezil, the medicine in Adlarity, are:
- nausea
- diarrhea
- not sleeping well
- vomiting
- muscle cramps
- feeling tired
- not wanting to eat
These are not all of the possible side effects of Adlarity.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA- 1088.
General information about the safe and effective use of Adlarity
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Adlarity for a condition for which it was not prescribed. Do not give Adlarity to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider for information about Adlarity that is written for health professionals.
How should I store Adlarity?
- Store Adlarity in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not store Adlarity in the freezer.
- Keep Adlarity in the sealed pouch until ready for use.
Keep Adlarity and all medicines out of the reach of children.
What are the ingredients in Adlarity?
Active ingredient: donepezil hydrochloride
Inactive ingredients: acrylate copolymer, ascorbyl palmitate, crospovidone, glycerol, lauryl lactate, polypropylene membrane, sodium bicarbonate, sorbitan monolaurate, and triethyl citrate.
For more information, go to www.ADLARITY.com, or call 1-800-910-8432.
Instructions for use for Adlarity
Adlarity (Ad-lare-it-ee)
(donepezil transdermal system)
Read this Instructions for Use before you use Adlarity for the first time and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition and treatment. Talk to your healthcare provider or pharmacist about how to use the Adlarity transdermal system.
Important information you need to know before using Adlarity:
- Use the Adlarity transdermal system exactly as your doctor tells you to.
- Wear only 1 transdermal system at a time.
- Each Adlarity transdermal system should be worn continuously for 7 days.
- The Adlarity transdermal system should be worn during showers or baths.
- Each Adlarity transdermal system should be replaced after 7 days. Choose a convenient time on a specific day of the week that works best for the regular application of the Adlarity transdermal system, such as every Saturday morning.
- Do not apply a new transdermal system to the same skin area for at least 2 weeks (14 days) after removal to decrease the chance of skin irritation. For example, a new transdermal system can be placed on the same body location, such as the back, but should not be placed on the same area of the back for at least 2 weeks.
- Remove the old transdermal system before applying the new transdermal system to a different skin site. You can write down the date and site of application to remind you when to remove and replace the transdermal system.
- Avoid exposure to heat sources such as excessive sunlight, saunas, sunrooms, or heating pads for long periods of time. Too much medicine could be absorbed into your body.
- Use Adlarity within 24 hours after removal from the refrigerator.
- Do not apply a cold transdermal system. Do not use external heat sources, such as a microwave, hair dryer, heating pad or direct sunlight, to warm the transdermal system.
The Adlarity transdermal system
- Adlarity is a tan rectangular transdermal system that comes in 2 strengths: 5 mg and 10 mg.
- Adlarity transdermal system is supplied in a carton containing 4 individual transdermal systems of the same strength (4 week supply). Each transdermal system is sealed inside a pouch protecting it until you are ready to apply it.
- Adlarity is applied 1 time weekly (every 7 days).
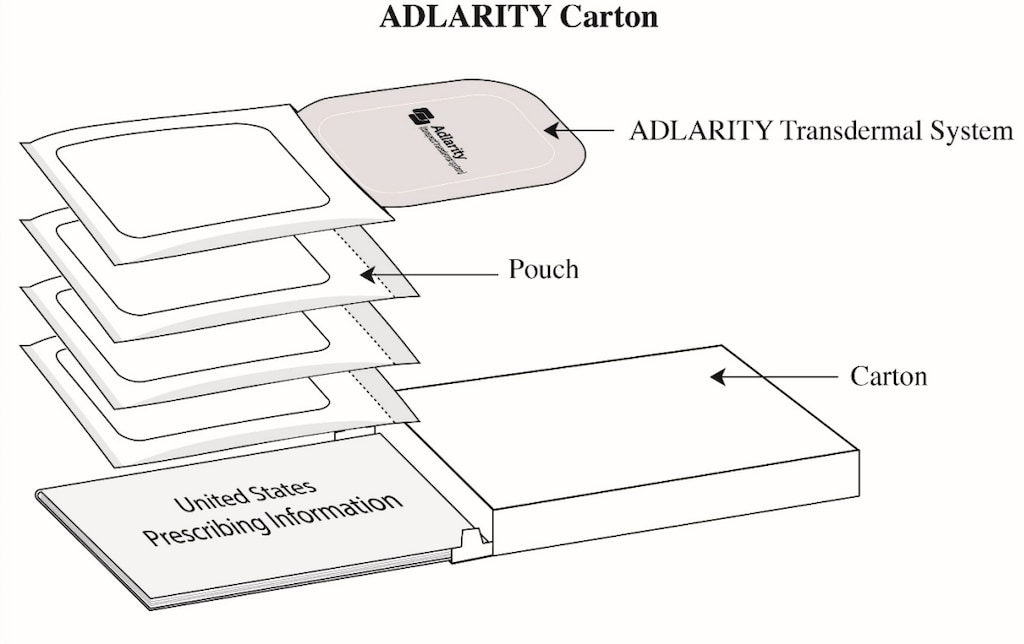
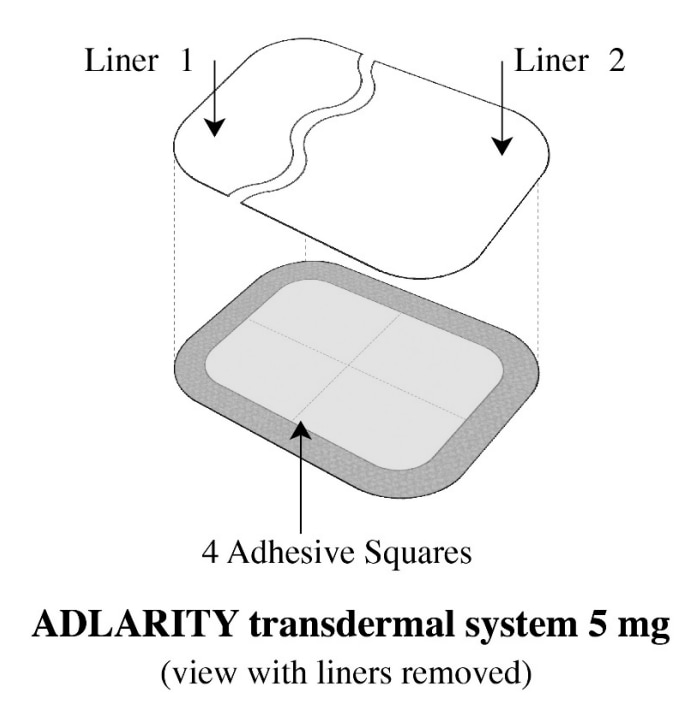
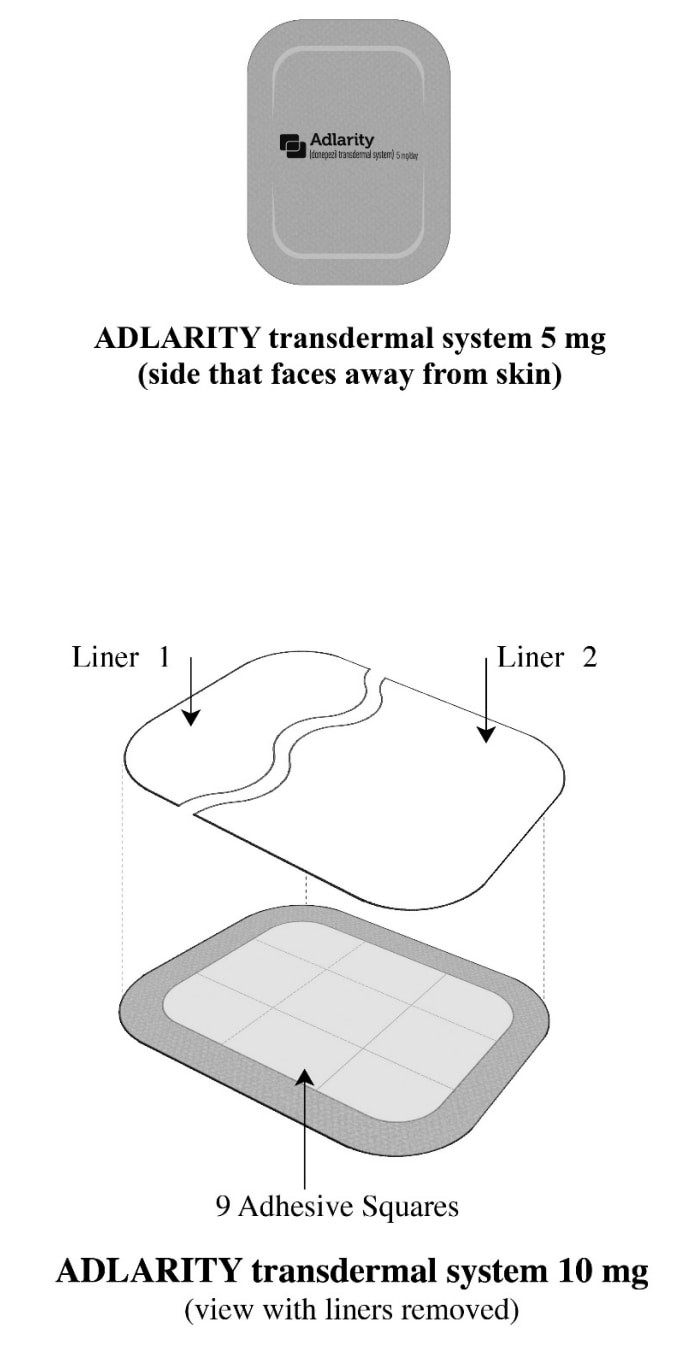

How to store Adlarity
- Store Adlarity in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not store Adlarity in the freezer.
- Keep Adlarity in the sealed pouch until ready for use.
- Keep Adlarity and all medicines out of the reach of children.
How to use Adlarity
Step 1: Where to apply the Adlarity transdermal system
Important: Wear only 1 transdermal system at a time.
- Check the expiration date.
- Wash your hands with soap and water before handling the transdermal system.
- Choose a clean and dry area of skin, where no cream, lotion, or powder has recently been applied.
- Choose skin without cuts or scrapes and hairless or nearly hairless. If there is hair at the skin site, do not shave as this may increase risk of skin irritation. Use scissors to clip hair as close to the skin as possible, before applying the transdermal system.
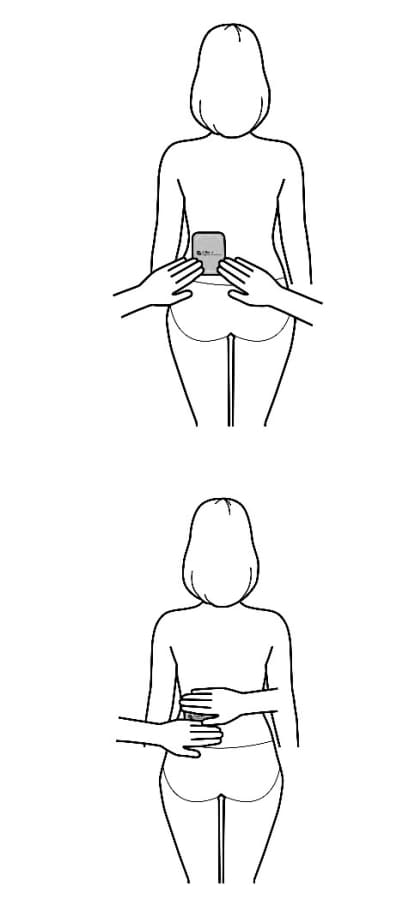
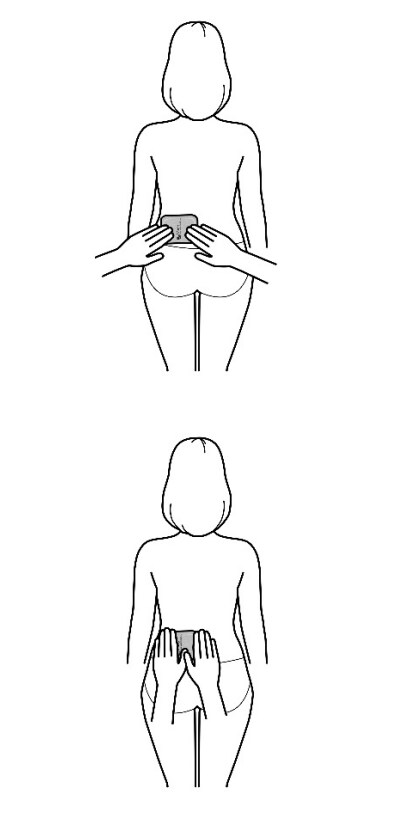
- Apply the transdermal system to the skin in areas shown with grey shading. The recommended application site is the back. If self-applying the transdermal system or if the transdermal system is not expected to be removed by the patient before the full 7 days, Adlarity can be placed on the upper buttocks or upper outer thigh.
- Apply the transdermal system in either a vertical (short side of the transdermal system at the top and bottom) or horizontal (short side of the transdermal system on the left and right) direction. If the transdermal system is placed on the buttocks, it should be positioned in a horizontal direction.
- Do not apply the transdermal system to skin that has cream, lotion, or powder on it.
- Do not apply the transdermal system to an area where it can be rubbed off by tight clothing or undergarments.
- Do not apply the transdermal system over skin folds or across the spine.
Application sites
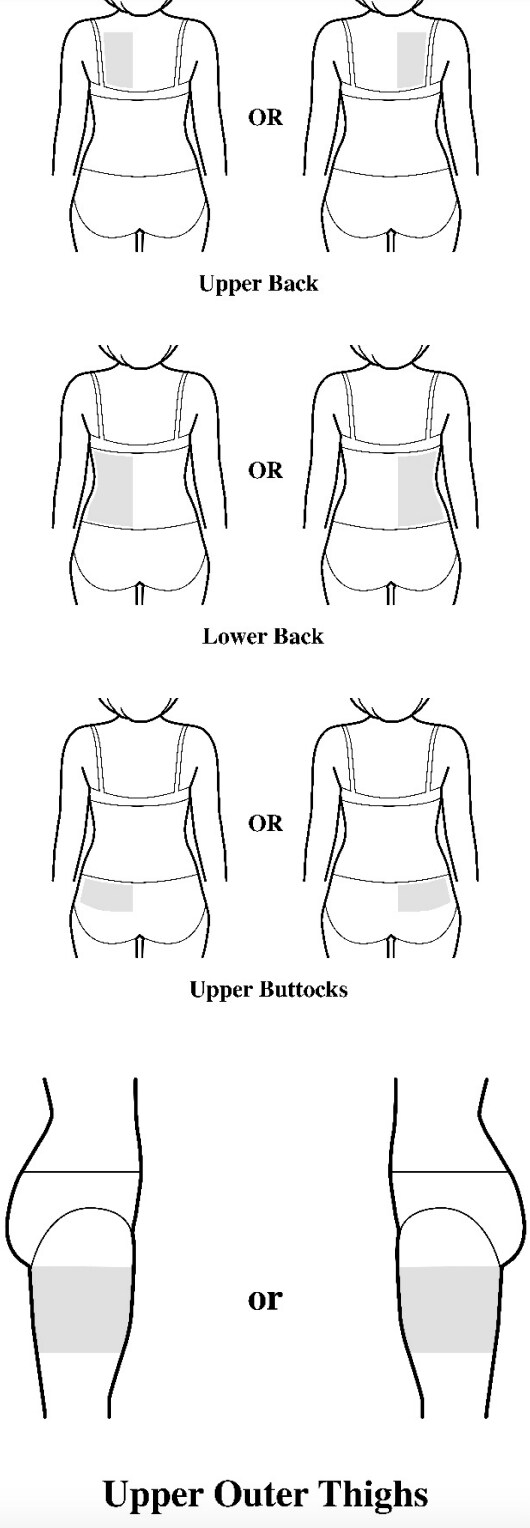
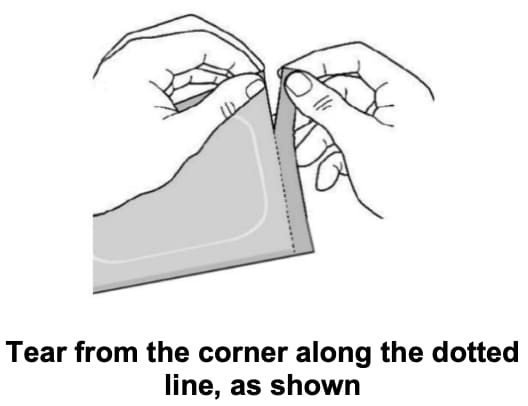
Step 2: Removing the Adlarity transdermal system from the pouch
- Allow the pouch to reach room temperature before opening and applying.
- Make sure seal is not broken.
- Open the pouch by carefully tearing along the dotted line from the corner.
- Remove the Adlarity transdermal system from the pouch. Apply to skin right away.
- Do not cut, damage, or use parts of the transdermal system. Use the full transdermal system to get required dose.
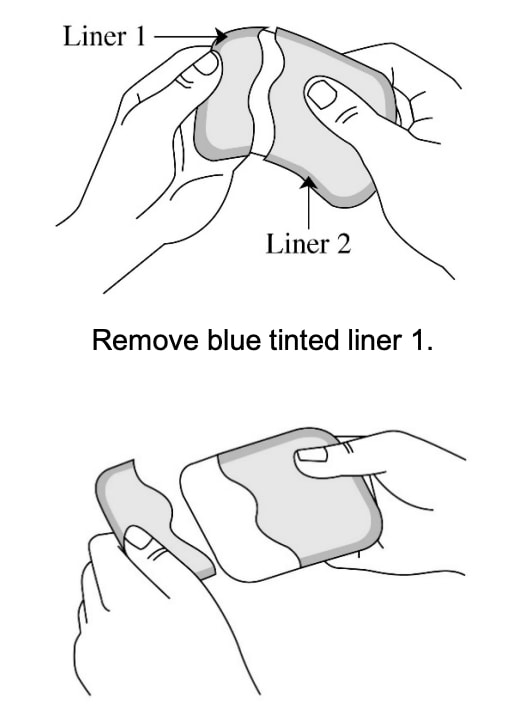
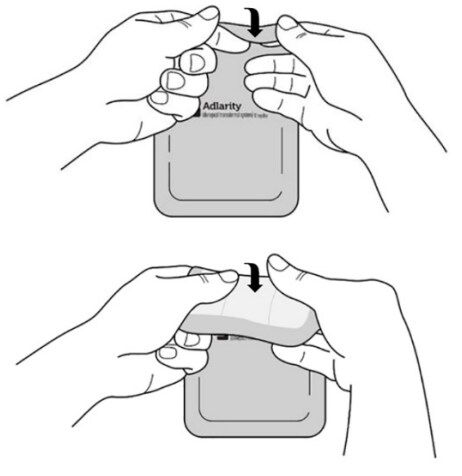
Step 3: Removing liner 1 from the Adlarity transdermal system
- Hold the transdermal system so the liner side (blue tinted transparent film) is facing you.
- Bend the transdermal system at the wavy cut in the middle to separate Liner 1 from Liner 2
- Remove Liner 1. Keep Liner 2 attached to the transdermal system (you will remove it in Step 4).
- Avoid touching the sticky (adhesive) side of the transdermal system with your fingers as that can keep the transdermal system from staying on for the full 7 days. Avoid sticking the transdermal. system to itself.
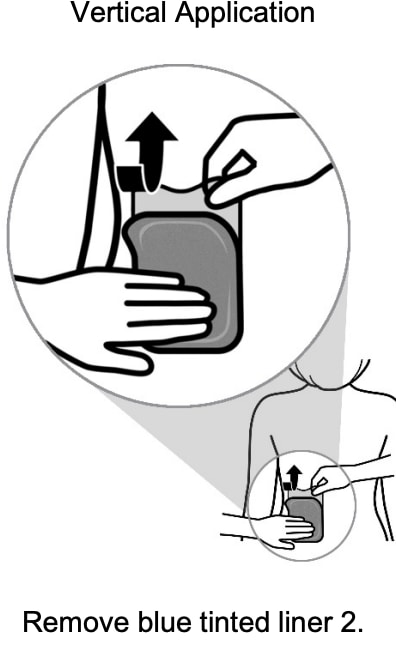
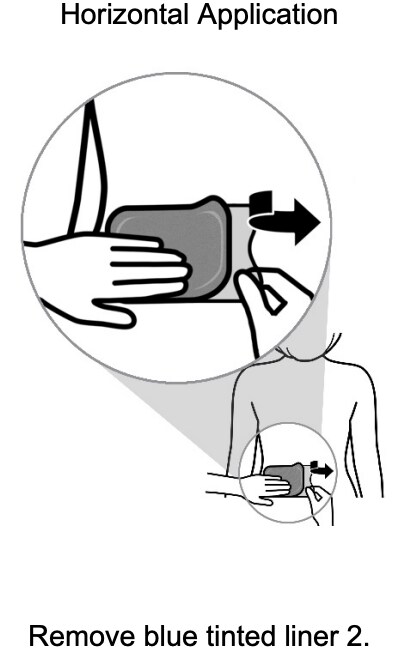
Step 4: Applying the Adlarity transdermal system and removing liner 2
- Place the short, sticky (adhesive) side of the transdermal system to the application site against the patient’s skin. Slowly peel away blue tinted liner 2 while applying gentle pressure with the fingers of your other hand to smooth the transdermal system in place.
- Smooth the entire transdermal system with your fingers or palm of your hand to avoid creating folds. Press down on the transdermal system firmly for 30 seconds to make sure that the edges stick to the skin.
- Wash your hands with soap and water after applying the transdermal system.
Step 5: Removing the Adlarity transdermal system
- Follow the steps below to remove and safely throw away (dispose of) a used Adlarity transdermal system after 7 days.
- Using the thumbs of both hands, slowly and evenly peel off the Adlarity transdermal system from the top to the bottom.
- Following this step may lower the chance that pieces of the transdermal system will be left on the skin and cause irritation at the application site.
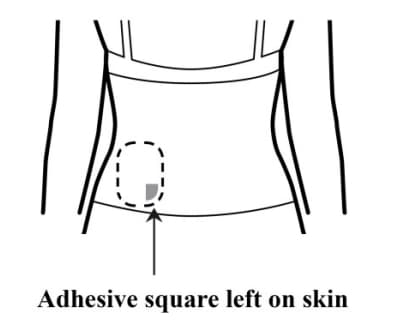
Step 6: Checking the skin for any remaining adhesive squares
- Look at the transdermal system application site for any remaining adhesive squares left on the skin.
- If you see any adhesive squares left on the skin, you can use your fingers to remove them and place them on the sticky side of the used transdermal system.
- Mineral oil or baby oil may be used to wipe off any remaining adhesive.
- Do not use alcohol or nail polish remover to remove remaining adhesive.
Step 7: Disposing of the used Adlarity transdermal system
- Fold the transdermal system in half with the sticky (adhesive) side pressed together.
- Throw away (dispose of) the used transdermal system in the household trash.
- Do not reuse transdermal systems.
- Do not flush used transdermal systems down the toilet.
- Wash your hands with soap and water after you throw away (dispose of) the used transdermal system.
Note: Some medicine stays in the transdermal system after the 7 days of use. The used transdermal system should be folded together and safely thrown away.
NOTE: If the transdermal system falls off before it is time to change it, do not try to reapply that transdermal system. Instead, choose a new application site and repeat Step 2 through Step 4 to apply a new transdermal system to the skin. Replace the new transdermal system 7 days later to start a new 1 week cycle.
For example, if you usually replace the Adlarity transdermal system on Saturdays, but during one of the weeks the transdermal system falls off on Wednesday, apply a new Adlarity transdermal system right away and replace it the following Wednesday.
Instructions for use approved 03/2022.




