Boxed Warning
Pancreatitis:
Fatal and nonfatal pancreatitis have occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of the degree of immunosuppression. Suspend didanosine in patients with suspected pancreatitis; discontinue didanosine in patients with confirmed pancreatitis.
Lactic acidosis/severe hepatomegaly:
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination, including didanosine and other antiretrovirals. Fatal lactic acidosis has been reported in pregnant individuals who received the combination of didanosine and stavudine with other antiretroviral agents. Coadministration of didanosine and stavudine is contraindicated because of increased risk of serious and/or life-threatening events. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Capsule Delayed Release, Oral:
Videx EC: 125 mg, 200 mg, 250 mg, 400 mg [DSC]
Generic: 125 mg [DSC], 200 mg, 250 mg, 400 mg
Solution Reconstituted, Oral:
Videx: 2 g (100 mL); 4 g (200 mL [DSC])
Pharmacology
Mechanism of Action
Didanosine, a purine nucleoside (adenosine) analog and the deamination product of dideoxyadenosine (ddA), inhibits HIV replication in vitro in both T cells and monocytes. Didanosine is converted within the cell to the mono-, di-, and triphosphates of ddA. These ddA triphosphates act as substrate and inhibitor of HIV reverse transcriptase substrate and inhibitor of HIV reverse transcriptase thereby blocking viral DNA synthesis and suppressing HIV replication.
Pharmacokinetics/Pharmacodynamics
Absorption
Subject to degradation by acidic pH of stomach; some formulations are buffered to resist acidic pH; ≤55% reduction in peak plasma concentration is observed in presence of food. Delayed release capsules contain enteric-coated beadlets which dissolve in the small intestine.
Distribution
Extensive intracellular distribution
CSF/plasma ratio: Infants 8 months to Adolescents 19 years: 46% (range: 12% to 85%); Adults: 21%
Vd (apparent):
Age-based:
Infants 8 months to Adolescents 19 years: 28 ± 15 L/m2
Adults: 43.7 ± 8.9 L/m2
Weight-based:
Children 20 kg to <25 kg: 98 ± 30 L
Children 25 kg to <60 kg: 155 ± 55 L
Children ≥60 kg: 363 ± 138 L
Adults ≥60 kg: 308 ± 164 L
Metabolism
Has not been evaluated in humans; studies conducted in dogs, show extensive metabolism with allantoin, hypoxanthine, xanthine, and uric acid being the major metabolites found in urine
Excretion
Unchanged drug excreted in urine
Infants 8 months to Adolescents 19 years: 18% ± 10%
Adults: 18% ± 8%
Time to Peak
Delayed release capsules: 2 hours; Powder for suspension: 0.25 to 1.5 hours
Half-Life Elimination
Plasma:
Newborns (1 day old): 2 ± 0.7 hours
Infants 2 weeks to 4 months: 1.2 ± 0.3 hours
Infants 8 months to Adolescents 19 years: 0.8 ± 0.3 hours
Adults with normal renal function: 1.5 ± 0.4 hours
Intracellular: Adults: 25 to 40 hours
Elimination: Increased as CrCl decreased
Children 20 kg to <25 kg: 0.75 ± 0.13 hours
Children 25 kg to <60 kg: 0.92 ± 0.09 hours
Children ≥60 kg: 1.26 ± 0.19 hours
Adults ≥60 kg: 1.19 ± 0.21 hours; 2 ± 0.3 hours (renal impairment [CrCl <30 mL/minute]); 4.1 ± 1.2 hours (dialysis)
Protein Binding
<5%
Use in Specific Populations
Special Populations: Renal Function Impairment
Half-life increased and clearance decreased as the CrCl decreased.
Special Populations: Hepatic Function Impairment
Mean Cmax and AUC were 19% and 13% higher in patients with moderate to severe hepatic impairment.
Use: Labeled Indications
HIV infection: Treatment of HIV-1 infection in combination with other antiretroviral agents.
Contraindications
Coadministration with allopurinol, ribavirin, or stavudine
Canadian labeling: Additional contraindications (not in US labeling): Hypersensitivity to didanosine or any component of the formulation
Dosage and Administration
Dosing: Adult
Treatment of HIV-1 infection: Oral:
Dosing based on patient weight:
Pediatric powder for oral solution:
<60 kg: 125 mg twice daily (preferred) or 250 mg once daily
≥60 kg: 200 mg twice daily (preferred) or 400 mg once daily
Delayed-release capsule:
20 kg to <25 kg: 200 mg once daily
25 kg to <60 kg: 250 mg once daily
≥60 kg: 400 mg once daily
Dosage adjustment for concomitant therapy:
When taken with tenofovir:
<60 kg and CrCl ≥60 mL/minute: 200 mg once daily
≥60 kg and CrCl ≥60 mL/minute: 250 mg once daily
Dosing: Geriatric
Refer to adult dosing. Elderly patients have a higher frequency of pancreatitis (10% versus 5% in younger patients); monitor renal function and dose accordingly.
Dosing: Pediatric
Note: Gene mutation and antiretroviral (ARV) resistance patterns should be evaluated (refer to www.iasusa.org for more information) when necessary.
HIV-1 infection, treatment: Oral: Note: Although FDA approved, didanosine is no longer recommended for use in children and adolescents due to higher rates of adverse effects than other nucleoside reverse transcriptase inhibitors (HHS [pediatric] 2018). If used, should be in combination with other ARV agents:
Infants 1 to 8 months (HHS [pediatric] 2018): Oral solution:
Infants 1 to <3 months: 50 mg/m2/dose every 12 hours. Note: Manufacturer's labeling not recommended (100 mg/m2/dose); pharmacokinetic data suggest toxicity may occur at this higher dose (HHS [pediatric] 2018).
Infants ≥3 to 8 months: 100 mg/m2/dose every 12 hours.
Infants >8 months, Children, and Adolescents:
Oral solution:
Twice-daily dosing: Infants >8 months, Children, and Adolescents: 120 mg/m2/dose (range: 90 to 150 mg/m2/dose) every 12 hours; do not exceed weight-based adult dose (HHS [pediatric] 2018; manufacturer labeling).
Once-daily dosing for treatment-naive patients: Children ≥3 year and Adolescents: 240 mg/m2/dose once daily; maximum dose: 400 mg/dose (HHS [pediatric] 2018).
Delayed-release capsule: Children ≥6 years weighing ≥20 kg who are able to swallow a capsule whole:
20 kg to <25 kg: 200 mg once daily.
25 kg to <60 kg: 250 mg once daily.
≥60 kg: 400 mg once daily.
Reconstitution
Pediatric powder for oral solution: Prior to dispensing, add 100 mL or 200 mL purified water, USP to the 2 g or 4 g container, respectively, to achieve a 20 mg/mL solution. Immediately mix the resulting solution with an equal volume of antacid that contains the active ingredients aluminum hydroxide (400 mg/5 mL), magnesium hydroxide (400 mg/5 mL) and simethicone (40 mg/5 mL) to achieve a final concentration of 10 mg/mL. Dispense in flint glass or plastic (eg, HDPE, PET or PETG) bottles with child resistant closures.
Administration
Oral: Pediatric powder for oral solution: Administer on an empty stomach at least 30 minutes before or 2 hours after eating. Shake well prior to use.
Oral: Videx EC: Administer on an empty stomach at least 30 minutes before or 2 hours after eating; swallow capsule whole.
Dietary Considerations
Take on an empty stomach; administer at least 30 minutes before or 2 hours after eating
Storage
Delayed release capsules should be stored in tightly closed bottles at controlled room temperature of 25°C (77°F). Unreconstituted powder should be stored at 15°C to 30°C (59°F to 86°F); reconstituted oral solution is stable for 30 days stored at 2°C to 8°C (36°F to 46°F).
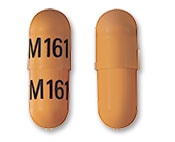
Didanosine Images
Drug Interactions
Alcohol (Ethyl): May enhance the adverse/toxic effect of Didanosine. Specifically, the risk of pancreatitis may be increased. Avoid combination
Allopurinol: May increase the serum concentration of Didanosine. Avoid combination
Antifungal Agents (Azole Derivatives, Systemic): Didanosine may decrease the absorption of Antifungal Agents (Azole Derivatives, Systemic). Enteric coated didanosine capsules are not expected to affect these antifungals. Exceptions: Isavuconazonium Sulfate. Consider therapy modification
Atazanavir: Didanosine may decrease the serum concentration of Atazanavir. Specifically, the buffered formulation of didanosine may decrease atazanavir absorption. Atazanavir may decrease the serum concentration of Didanosine. Reported with enteric coated didanosine capsules. Management: To avoid therapeutic failure of atazanavir the drug should be administered 2 hours before or 1 hour after didanosine. This recommendation applies to both buffered didanosine products and enteric coated didanosine products. Consider therapy modification
Cladribine: Agents that Undergo Intracellular Phosphorylation may diminish the therapeutic effect of Cladribine. Avoid combination
Darunavir: May decrease the serum concentration of Didanosine. More specifically, this interaction is likely due to the effects of food (with which darunavir/ritonavir and darunavir/cobicistat are taken) on didanosine, which is supposed to be given on an empty stomach. Management: Didanosine should be administered 1 hour prior to or 2 hours after administration of darunavir/ritonavir or darunavir/cobicistat (which must be taken with food). Consider therapy modification
Febuxostat: May increase the serum concentration of Didanosine. Avoid combination
Ganciclovir-Valganciclovir: May increase the serum concentration of Didanosine. Monitor therapy
Hydroxyurea: Didanosine may enhance the adverse/toxic effect of Hydroxyurea. An increased risk of pancreatitis, hepatotoxicity and/or neuropathy may exist. Hydroxyurea may enhance the adverse/toxic effect of Didanosine. An increased risk of pancreatitis, hepatotoxicity and/or neuropathy may exist. Avoid combination
Indinavir: Didanosine may decrease the serum concentration of Indinavir. Management: Indinavir should be administered at least 1 hour apart from buffer-containing formulations of didanosine. Consider therapy modification
Levomethadone: May decrease the serum concentration of Didanosine. Monitor therapy
Lopinavir: May decrease the serum concentration of Didanosine. This interaction refers only to lopinavir/ritonavir oral solution, which must be taken with food, and is principally the result of a food-didanosine interaction. Management: Didanosine should be administered 1 hour prior to or 2 hours after administration of lopinavir/ritonavir oral solution (which must be taken with food). Didanosine and lopinavir/ritonavir tablets can be administered together. Consider therapy modification
Methadone: May decrease the serum concentration of Didanosine. Monitor therapy
Orlistat: May decrease the serum concentration of Antiretroviral Agents. Monitor therapy
Quinolones: May decrease the serum concentration of Didanosine. Didanosine may decrease the serum concentration of Quinolones. Management: Administer oral quinolones at least 2 hours before or 6 hours after didanosine. Monitor for decreased therapeutic effects of quinolones, particularly if doses cannot be separated as recommended. This does not apply to unbuffered enteric coated didanosine. Exceptions: LevoFLOXacin (Oral Inhalation). Consider therapy modification
Ribavirin (Oral Inhalation): May enhance the adverse/toxic effect of Didanosine. Ribavirin (Oral Inhalation) may increase serum concentrations of the active metabolite(s) of Didanosine. Avoid combination
Ribavirin (Systemic): May enhance the adverse/toxic effect of Didanosine. Ribavirin (Systemic) may increase serum concentrations of the active metabolite(s) of Didanosine. Avoid combination
Rilpivirine: May decrease the absorption of Didanosine. More specifically, simultaneous coadministration of these drugs creates a conflict between recommendations to administer with (rilpivirine) and without (didanosine) food. Didanosine may decrease the absorption of Rilpivirine. More specifically, simultaneous coadministration of these drugs creates a conflict between recommendations to administer with (rilpivirine) and without (didanosine) food. Management: Administer didanosine on an empty stomach at least 2 hours before or 4 hours after rilpivirine, due to the requirement that rilpivirine be administered with food. Consider therapy modification
Stavudine: May enhance the adverse/toxic effect of Didanosine. The risk of lactic acidosis (possibly fatal), hepatomegaly, and pancreatitis may be increased with this combination. Avoid combination
Tenofovir Disoproxil Fumarate: May diminish the therapeutic effect of Didanosine. Tenofovir Disoproxil Fumarate may increase the serum concentration of Didanosine. Management: Avoid concomitant treatment with tenofovir disoproxil fumarate and didanosine. Consider altering even existing, stable treatment to avoid this combination. Avoid combination
Tipranavir: May decrease the serum concentration of Didanosine. Management: It is recommended that didanosine be administered at least 2 hours apart from tipranavir in order to minimize any potential dosage form-related interaction. Consider therapy modification
Adverse Reactions
As reported in monotherapy studies; risk of toxicity may increase when combined with other agents.
>10%:
Central nervous system: Peripheral neuropathy (17% to 20%)
Endocrine & metabolic: Increased amylase (≥1.4 x ULN: 15% to 17%)
Gastrointestinal: Diarrhea (19% to 28%), abdominal pain (7% to 13%)
1% to 10%:
Dermatologic: Pruritus (≤9%), skin rash (≤9%)
Endocrine & metabolic: Increased uric acid (>12 mg/dL: 2% to 3%)
Gastrointestinal: Pancreatitis (6% to 7%)
Hepatic: Increased serum AST (>5 x ULN: 7% to 9%), increased serum ALT (>5 x ULN: 6% to 9%), increased serum alkaline phosphatase (>5 x ULN: 1% to 4%)
<1%, postmarketing, and/or case reports: Acute renal failure, alopecia, anaphylactoid reaction, anemia, anorexia, arthralgia, chills, diabetes mellitus, dyspepsia, fever, flatulence, hepatic failure, hepatitis, hyperglycemia, hypoglycemia, increased creatine phosphokinase, increased gamma-glutamyl transferase, lactic acidosis, leukopenia, lipoatrophy (buttocks, face, limbs), myalgia, myopathy, optic neuritis, pain, parotid gland enlargement, portal hypertension (noncirrhotic), retinal pigment changes (depigmentation), rhabdomyolysis, severe hepatomegaly with steatosis, sialadenitis, symptomatic hyperlactatemia, thrombocytopenia, weakness, xerophthalmia, xerostomia
Warnings/Precautions
Concerns related to adverse effects:
- Immune reconstitution syndrome: Patients may develop immune reconstitution syndrome resulting in the occurrence of an inflammatory response to an indolent or residual opportunistic infection during initial HIV treatment or activation of autoimmune disorders (eg, Graves’ disease, polymyositis, Guillain-Barré syndrome) later in therapy; further evaluation and treatment may be required.
- Lactic acidosis/hepatomegaly: [US Boxed Warning]: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported, with nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. Risk may be increased with female gender, obesity, or prolonged exposure. Fatal lactic acidosis has been reported in pregnant individuals who received the combination of didanosine and stavudine with other antiretroviral agents. Use caution when administering to patients with known risk factors for liver disease. Suspend treatment in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or hepatotoxicity (transaminase elevation may/may not accompany hepatomegaly and steatosis).
- Lipoatrophy: May cause loss of subcutaneous fat, especially in the face, limbs, and buttocks. Lipoatrophy incidence and severity are related to cumulative exposure and may be only partially reversible. Monitor patients for signs of lipoatrophy and consider switching to a non-didanosine-containing regimen if lipoatrophy occurs.
- Noncirrhotic portal hypertension: Patients may develop noncirrhotic portal hypertension within months to years of starting didanosine therapy. Signs may include elevated liver enzymes, esophageal varices, hematemesis, ascites, and splenomegaly. Noncirrhotic portal hypertension may lead to liver failure and/or death. Discontinue use in patients with evidence of this condition.
- Ocular effects: Retinal changes (including retinal depigmentation) and optic neuritis have been reported in adults and children using didanosine; patients should undergo retinal examination periodically.
- Pancreatitis: [US Boxed Warning]: Pancreatitis (fatal and nonfatal) has been reported alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Suspend use in patients with suspected pancreatitis and discontinue in patients with confirmed pancreatitis; frequency is dose related. In patients with risk factors for pancreatitis, use with extreme caution and only if clearly indicated. Patients with advanced HIV-1 infection, especially the elderly, are at increased risk and should be followed closely. Patients with renal impairment may be at greater risk for pancreatitis if treated without dose adjustment.
- Peripheral neuropathy: Peripheral neuropathy (numbness, tingling or pain in the hands or feet) has been reported, more frequently in patients with advanced HIV disease, in patients with a history of neuropathy or in patients being treated with a neurotoxic drug. Discontinue therapy if neuropathy occurs.
Disease-related concerns:
- Hepatic impairment: Use with caution in patients with hepatic impairment; safety and efficacy have not been established in patients with significant hepatic disease. Patients on combination antiretroviral therapy with hepatic impairment may be at increased risk of potentially severe and fatal hepatic toxicity; consider interruption or discontinuation of therapy if hepatic impairment worsens.
- Renal impairment: Use with caution in patients with renal impairment; dose reduction recommended for CrCl <60 mL/minute.
Concurrent drug therapy issues:
- Hydroxyurea and stavudine: Fatal cases of hepatotoxicity/lactic acidosis, pancreatitis, and/or severe peripheral neuropathy have been reported in HIV patients treated with didanosine with hydroxyurea and stavudine; avoid use with hydroxyurea; coadministration with stavudine is contraindicated.
- Tenofovir: Combined use may be associated with increased didanosine toxicity (eg, lactic acidosis, pancreatitis), immunologic nonresponse or CD4 cell decline despite viral suppression, early virologic failure and rapid resistance development; combined use is not recommended (HHS [adult], 2015); manufacturer labeling recommends a didanosine dose reduction if combination is used.
- Drug-drug interactions: Additional potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Dosage form specific issues:
- Delayed-release capsules: Didanosine delayed-release capsules are indicated for once-daily use.
- Powder for oral solution: Didanosine powder for oral solution is recommended for use in a twice daily regimen, as there is more efficacy evidence with twice daily administration.
Special populations:
Pediatric: Dosing recommendations for didanosine powder for oral solution in patients younger than 2 weeks cannot be made because the pharmacokinetics of didanosine in these infants are too variable to determine an appropriate dose. Delayed-release capsules may be used in pediatric patients who weigh at least 20 kg.
Monitoring Parameters
Serum potassium, uric acid, creatinine; hemoglobin, CBC with neutrophil and platelet count, CD4 cells; viral load; liver function tests, serum bilirubin, albumin, INR, amylase; weight gain; perform dilated retinal exam every 6 months, ultrasonography (if portal hypertension suspected)
Pregnancy
Pregnancy Considerations
[US Boxed Warning]: Fatal lactic acidosis has been reported in pregnant individuals using didanosine and stavudine in combination with other antiretroviral agents. The Health and Human Services (HHS) perinatal HIV guidelines do not recommend didanosine use in pregnant women due to toxicity, and women who are pregnant should be changed to a preferred or alternative therapy.
Didanosine crosses the human placenta.
Outcome information specific to didanosine use in pregnancy is no longer being reviewed and updated in the HHS perinatal guidelines. Maternal antiretroviral therapy (ART) may be associated with adverse pregnancy outcomes including preterm delivery, stillbirth, low birth weight, and small for gestational age infants. Actual risks may be influenced by maternal factors, such as disease severity, gestational age at initiation of therapy, and specific ART regimen, therefore close fetal monitoring is recommended. Because there is clear benefit to appropriate treatment, maternal ART should not be withheld due to concerns for adverse neonatal outcomes. Long-term follow-up is recommended for all infants exposed to antiretroviral medications; children without HIV but who were exposed to ART in utero and develop significant organ system abnormalities of unknown etiology (particularly of the CNS or heart) should be evaluated for potential mitochondrial dysfunction. Cases of lactic acidosis and hepatic steatosis have been reported in pregnant women with use of nucleoside reverse transcriptase inhibitors.
In general, ART is recommended for all pregnant females living with HIV to keep the viral load below the limit of detection and reduce the risk of perinatal transmission. Therapy should be individualized following a discussion of the potential risks and benefits of treatment during pregnancy. Monitoring of pregnant females is more frequent than in nonpregnant adults. ART should be continued postpartum for all females living with HIV and can be modified after delivery.
Health care providers are encouraged to enroll pregnant females exposed to antiretroviral medications as early in pregnancy as possible in the Antiretroviral Pregnancy Registry (1-800-258-4263 or http://www.APRegistry.com). Health care providers caring for pregnant females living with HIV and their infants may contact the National Perinatal HIV Hotline (888-448-8765) for clinical consultation (HHS [perinatal] 2019).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience headache, abdominal pain, nausea, vomiting, or diarrhea. Have patient report immediately to prescriber signs of lactic acidosis (fast breathing, fast heartbeat, abnormal heartbeat, vomiting, fatigue, shortness of breath, severe loss of strength and energy, severe dizziness, feeling cold, or muscle pain or cramps), signs of pancreatitis (severe abdominal pain, severe back pain, severe nausea, or vomiting), signs of liver problems (dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin), abdominal edema, severe dizziness, passing out, burning or numbness feeling, bruising, bleeding, vision changes, change in body fat, or signs of infection (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.