Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Suspension, Oral, as acetate:
Megace ES: 625 mg/5 mL (150 mL) [contains alcohol, usp, sodium benzoate; lemon-lime flavor]
Megace Oral: 40 mg/mL (240 mL [DSC])
Generic: 40 mg/mL (10 mL, 240 mL, 480 mL); 400 mg/10 mL (10 mL); 625 mg/5 mL (150 mL)
Tablet, Oral, as acetate:
Generic: 20 mg, 40 mg
Pharmacology
Mechanism of Action
A synthetic progestin with antiestrogenic properties which disrupt the estrogen receptor cycle. Megestrol interferes with the normal estrogen cycle and results in a lower LH titer. May also have a direct effect on the endometrium. Megestrol is an antineoplastic progestin thought to act through an antileutenizing effect mediated via the pituitary. May stimulate appetite by antagonizing the metabolic effects of catabolic cytokines.
Pharmacokinetics/Pharmacodynamics
Absorption
Well absorbed
Metabolism
Hepatic (to free steroids and glucuronide conjugates)
Excretion
Urine (57% to 78%; 5% to 8% as metabolites); feces (8% to 30%) within 10 days
Onset of Action
Breast or endometrial cancer: At least 2 months of continuous therapy; Weight gain: 2 to 4 weeks
Time to Peak
Serum: Suspension: 5 hours; Tablet: 2.2 hours (range: 1 to 3 hours)
Half-Life Elimination
Suspension: 20 to 50 hours; Tablet: Mean: 34.2 hours (range: 13 to 105 hours)
Use: Labeled Indications
Anorexia or cachexia: Suspension: Treatment of anorexia, cachexia, or unexplained significant weight loss in patients with AIDS
Limitations of use: Treatment of AIDS-related weight loss should only be initiated after addressing the treatable causes (eg, malignancy, infection, malabsorption, endocrine disease, renal disease, psychiatric disorder) for weight loss. Megestrol is not intended to prevent weight loss.
Breast cancer: Tablet: Treatment (palliative) of advanced breast cancer
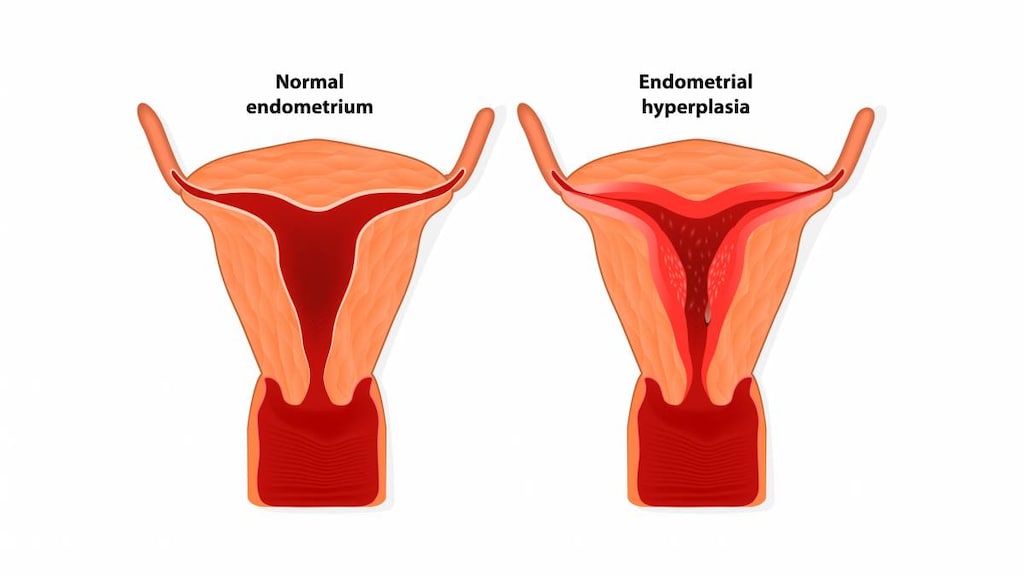
Endometrial cancer: Tablet: Treatment (palliative) of advanced endometrial carcinoma
Use: Off Label
Treatment of cancer-related cachexiaa
Data from randomized, placebo controlled studies Loprinzi 1990, Vadell 1998; support the use of megestrol to promote weight gain in cachectic patients with cancer. A meta-analysis of megestrol for the management of anorexia/cachexia showed benefit with the use of megestrol; higher doses were associated with more weight gain, however, data was insufficient to determine an optimal dose Ruiz Garcia 2013. Data from dose finding studies suggest higher doses are associated with higher weight gain Beller 1997, Loprinzi 1993.
Contraindications
Hypersensitivity to megestrol or any component of the formulation; known or suspected pregnancy (suspension).
Documentation of allergenic cross-reactivity for progestins is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Dosage and Administration
Dosing: Adult
Note: Megace ES suspension is not equivalent to other formulations on a mg-per-mg basis.
Anorexia or cachexia associated with AIDS: Oral: Suspension: Initial: 625 mg daily (of the 125 mg/mL suspension) or 800 mg daily (of the 40 mg/mL suspension); daily doses of 400 mg to 800 mg have been found to be effective
Breast cancer, advanced: Oral: Tablet: 160 mg per day in divided doses of 40 mg 4 times daily for at least 2 months
Endometrial cancer, advanced: Oral: Tablet: 40 to 320 mg daily in divided doses for at least 2 months
Cancer-related cachexia (off-label use): Oral: Doses ranging from 160 to 800 mg per day were effective in achieving weight gain, higher doses (>160 mg) were associated with more weight gain (Beller 1997; Loprinzi 1990; Loprinzi 1993; Vadell 1998); based on a meta-analysis, an optimal dose has not been determined (Ruiz Garcia 2013)
Dosing: Geriatric
Avoid use (Beers Criteria [AGS 2019]).
Dosing: Pediatric
Note: Megace ES is not equivalent mg per mg with other megestrol formulations.
Appetite stimulant/anorexia associated with chronic illness (eg, cancer, cystic fibrosis, HIV): Limited data available: Infants ≥8 months, Children, and Adolescents: Oral: Tablets or 40 mg/mL suspension: Initial dose: 7.5 to 10 mg/kg/day in 1 to 2 divided doses; titrate dose to response, decrease dose if weight gain is excessive. Reported duration was 3 to 11 months. Maximum daily dose: 800 mg/day or 15 mg/kg/day
Dosing based on several prospective and retrospective trials and case series. Clinical trials show benefit for weight gain and body mass (Azcona 1996; Brady 1994; Clarick 1997; Cuvelier 2014; Eubanks 2002; Hobbs 2010; Marchand 2000; Nasr 1999; Orme 2003).
Administration
Oral: Shake suspension well before use.
The 625 mg/5 mL suspension may be administered without regard to meals.
Storage
Suspension: Store at 15°C to 25°C (59°F to 77°F); protect from heat. Store/dispense in a tight container.
Tablet: Store at 15°C to 30°C (59°F to 86°F); protect from light. Protect from temperatures above 40°C (104°F).
Megestrol Images
Drug Interactions
Anticoagulants: Progestins may diminish the therapeutic effect of Anticoagulants. More specifically, the potential prothrombotic effects of some progestins and progestin-estrogen combinations may counteract anticoagulant effects. Management: Carefully weigh the prospective benefits of progestins against the potential increased risk of procoagulant effects and thromboembolism. Use is considered contraindicated under some circumstances. Refer to related guidelines for specific recommendations. Consider therapy modification
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
C1 inhibitors: Progestins may enhance the thrombogenic effect of C1 inhibitors. Monitor therapy
Choline C 11: Antiandrogens may diminish the therapeutic effect of Choline C 11. Monitor therapy
Dofetilide: Megestrol may increase the serum concentration of Dofetilide. Avoid combination
Herbs (Progestogenic Properties) (eg, Bloodroot, Yucca): May enhance the adverse/toxic effect of Progestins. Monitor therapy
Indium 111 Capromab Pendetide: Antiandrogens may diminish the diagnostic effect of Indium 111 Capromab Pendetide. Avoid combination
Pomalidomide: Progestins may enhance the thrombogenic effect of Pomalidomide. Management: Canadian pomalidomide labeling recommends caution with use of hormone replacement therapy and states that hormonal contraceptives are not recommended. US pomalidomide labeling does not contain these specific recommendations. Consider therapy modification
Ulipristal: Progestins may diminish the therapeutic effect of Ulipristal. Ulipristal may diminish the therapeutic effect of Progestins. Management: Ulipristal for uterine fibroids (Canadian indication): avoid progestins within 12 days of stopping ulipristal; as emergency contraceptive (U.S. indication): avoid progestins within 5 days of stopping ulipristal. Avoid combination
Adverse Reactions
Frequency not always defined.
Cardiovascular: Hypertension (4% to 8%), cardiomyopathy (1% to 3%), chest pain (1% to 3%), edema (1% to 3%), palpitations (1% to 3%), peripheral edema (1% to 3%), cardiac failure
Central nervous system: Headache (3% to 10%), pain (4% to 6%, similar to placebo), insomnia (1% to 6%), abnormality in thinking (1% to 3%), confusion (1% to 3%), convulsions (1% to 3%), depression (1% to 3%), hypoesthesia (1% to 3%), neuropathy (1% to 3%), paresthesia (1% to 3%), carpal tunnel syndrome, lethargy, malaise, mood changes
Dermatologic: Skin rash (6% to 12%), alopecia (1% to 3%), dermatological disease (1% to 3%), diaphoresis (1% to 3%), pruritus (1% to 3%), vesicobullous dermatitis (1% to 3%)
Endocrine & metabolic: Hyperglycemia (6%), decreased libido (1% to 5%), albuminuria (1% to 3%), gynecomastia (1% to 3%), increased lactate dehydrogenase (1% to 3%), adrenocortical insufficiency, amenorrhea, Cushing's syndrome, diabetes mellitus, hot flash, HPA-axis suppression, hypercalcemia, weight gain (not attributed to edema or fluid retention)
Gastrointestinal: Diarrhea (10%, similar to placebo), flatulence (6% to 10%), vomiting (4% to 6%), nausea (4% to 5%), dyspepsia (2% to 3%), abdominal pain (1% to 3%), constipation (1% to 3%), oral moniliasis (1% to 3%), sialorrhea (1% to 3%), xerostomia (1% to 3%)
Genitourinary: Impotence (4% to 14%), urinary incontinence (1% to 3%), urinary tract infection (1% to 3%), urinary frequency (1% to 2%), breakthrough bleeding
Hematologic & oncologic: Leukopenia (1% to 3%), sarcoma (1% to 3%), tumor flare
Hepatic: Hepatomegaly (1% to 3%)
Infection: Candidiasis (1% to 3%), herpes virus infection (1% to 3%), infection (1% to 3%)
Neuromuscular & skeletal: Weakness (5% to 6%)
Ophthalmic: Amblyopia (1% to 3%)
Respiratory: Cough (1% to 3%), dyspnea (1% to 3%), pharyngitis (1% to 3%), pulmonary disorder (1% to 3%), pneumonia (1%), hyperventilation
Miscellaneous: Fever (1% to 6%)
Postmarketing and/or case reports: Decreased glucose tolerance, thromboembolic phenomena (including deep vein thrombosis, pulmonary embolism, thrombophlebitis)
Warnings/Precautions
Concerns related to adverse effects:
- Adrenal suppression: May suppress hypothalamic-pituitary-adrenal (HPA) axis during chronic administration; consider the possibility of adrenal suppression in any patient receiving or being withdrawn from chronic therapy when signs/symptoms suggestive of hypoadrenalism are noted (during stress or in unstressed state). Laboratory evaluation and replacement/stress doses of rapid-acting glucocorticoid should be considered.
- Cushing syndrome: Has been reported with long-term use.
Disease-related concerns:
- AIDS-related cachexia: The effects on HIV viral replications are unknown.
- Diabetes: New-onset diabetes mellitus and exacerbation of preexisting diabetes have been reported with long-term use.
- Thromboembolism: Use with caution in patients with a history of thromboembolic disease.
Special populations:
- Females: Vaginal bleeding or discharge may occur.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant drug interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Dosage form specific issues:
- Benzyl alcohol and derivatives: Some dosage forms may contain sodium benzoate/benzoic acid; benzoic acid (benzoate) is a metabolite of benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol derivative with caution in neonates. See manufacturer’s labeling.
- Concentrated suspension: Megace ES suspension in not equivalent to other formulations on a mg-per-mg basis; Megace ES suspension 625 mg/5 mL is equivalent to megestrol acetate suspension 800 mg/20 mL.
Monitoring Parameters
Observe for signs of thromboembolic events; blood pressure, weight; serum glucose
Pregnancy
Pregnancy Considerations
Use is contraindicated for the treatment of anorexia or cachexia in pregnant females with HIV infection.
Megestrol may cause fetal harm if administered during pregnancy.
Evaluate pregnancy status prior to treatment in females of reproductive potential. Effective contraception should be used when treating anorexia or cachexia in females with HIV infection. In clinical studies, megestrol was shown to cause breakthrough vaginal bleeding in women.
Patient Education
What is this drug used for?
- It is used to help raise feelings of hunger.
- It is used to treat endometrial or breast cancer.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Increased hunger
- Trouble sleeping
- Hair loss
- Hot flashes
- Sweating a lot
- Diarrhea
- Passing gas
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- High blood sugar like confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit
- Cushing syndrome like weight gain in upper back or abdomen; moon face; severe headache; or slow healing
- Adrenal gland problems like severe nausea, vomiting, severe dizziness, passing out, muscle weakness, severe fatigue, mood changes, lack of appetite, or weight loss
- Severe nausea
- Vomiting
- Shortness of breath
- Excessive weight gain
- Swelling of arms or legs
- Abnormal vaginal bleeding
- Mood changes
- Sexual dysfunction
- Decreased sex drive
- Severe headache
- Severe dizziness
- Passing out
- Severe loss of strength and energy
- Vision changes
- Blood clots like numbness or weakness on one side of the body; pain, redness, tenderness, warmth, or swelling in the arms or legs; change in color of an arm or leg; chest pain; shortness of breath; fast heartbeat; or coughing up blood
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.