Boxed Warning
QT Prolongation and Sudden Deaths:
Nilotinib prolongs the QT interval. Prior to nilotinib administration and periodically, monitor for hypokalemia or hypomagnesemia and correct deficiencies. Obtain ECGs to monitor the QTc at baseline, 7 days after initiation, and periodically thereafter, and following any dose adjustments. Sudden deaths have been reported in patients receiving nilotinib. Do not administer nilotinib to patients with hypokalemia, hypomagnesemia, or long QT syndrome. Avoid use of concomitant drugs known to prolong the QT interval and strong CYP3A4 inhibitors. Avoid food 2 hours before and 1 hour after taking a nilotinib dose.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Capsule, Oral:
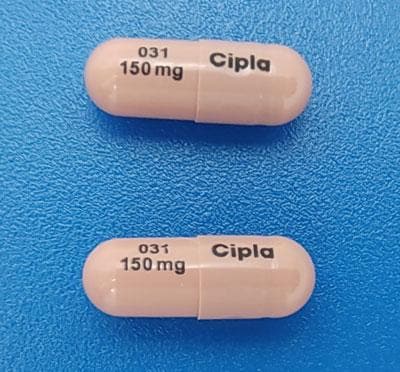
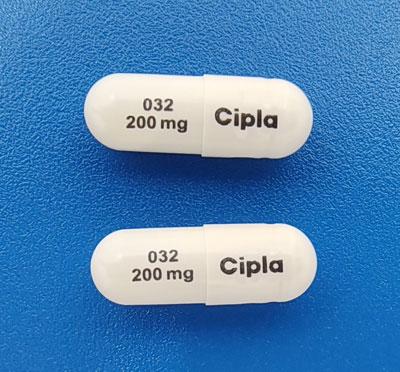
Tasigna: 50 mg, 150 mg, 200 mg
Pharmacology
Mechanism of Action
Nilotinib is a selective tyrosine kinase inhibitor that targets BCR-ABL kinase, c-KIT and platelet derived growth factor receptor (PDGFR); it does not have activity against the SRC family. Nilotinib inhibits BCR-ABL mediated proliferation of leukemic cell lines by binding to the ATP-binding site of BCR-ABL and inhibiting tyrosine kinase activity. Nilotinib has activity in imatinib-resistant BCR-ABL kinase mutations.
Pharmacokinetics/Pharmacodynamics
Metabolism
Hepatic; oxidation and hydroxylation, via CYP3A4 to primarily inactive metabolites
Excretion
Feces (93%; 69% as parent drug)
Time to Peak
3 hours
Half-Life Elimination
~17 hours
Protein Binding
~98%
Use in Specific Populations
Special Populations: Hepatic Function Impairment
Following a single 200 mg dose, the mean AUC increased 1.4-fold in patients with Child-Pugh classes A and B impairment and 1.6-fold in patients with Child Pugh class C impairment compared to subjects with normal hepatic function.
Special Populations Note
Total gastrectomy: Median steady-state trough concentrations are decreased by 53% (compared to patients who had not undergone gastric surgeries).
Use: Labeled Indications
Chronic myelogenous leukemia:
Treatment of newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia (CML) in chronic phase in pediatric patients ≥1 year and adults.
Treatment of chronic- and accelerated-phase Philadelphia chromosome-positive CML in adults resistant or intolerant to prior therapy that included imatinib.
Treatment of chronic phase Philadelphia chromosome-positive CML in pediatric patients ≥1 year with resistance or intolerance to prior tyrosine-kinase inhibitor therapy.
Use: Off Label
Acute lymphoblastic leukemia (ALL), Philadelphia chromosome-positive (Ph+)b
Data from a phase 2 trial support the use of nilotinib (in combination with chemotherapy and allogeneic stem cell transplant [in eligible patients]) for the treatment of newly diagnosed Ph+ ALL Kim 2015. Data from another small phase 2 trial support the use of nilotinib monotherapy in adult patients with relapsed/refractory Ph+ ALL following failure of imatinib and prior ALL chemotherapy Ottmann 2013.
Gastrointestinal stromal tumor (GIST) (refractory)a
Data from a randomized, phase III, multicenter, open-label study support the use of nilotinib in the treatment of GIST refractory to imatinib and sunitinib. Nilotinib provided a significantly longer median overall survival Reichardt 2012.
Contraindications
Hypokalemia, hypomagnesemia, or long QT syndrome
Canadian labeling: Additional contraindications (not in the US labeling): Hypersensitivity to nilotinib or any component of the formulation; persistent QTc >480 msec
Dosage and Administration
Dosing: Adult
Note: If clinically indicated, may be administered in combination with hematopoietic growth factors (eg, erythropoietin, filgrastim) and with hydroxyurea or anagrelide.
Acute lymphoblastic leukemia (ALL), Philadelphia chromosome-positive (Ph+), newly diagnosed (off-label use): Oral: 400 mg twice daily starting on day 8 of induction chemotherapy (in combination with daunorubicin, vincristine, and prednisolone); continue up to the start of stem cell transplant conditioning or until the end of consolidation therapy. Patients who completed 5 cycles of consolidation treatment received 2 years of nilotinib maintenance. Patients who underwent allogeneic stem cell transplant did not receive nilotinib after transplant (Kim 2015).
ALL, Ph+, relapsed/refractory (off-label use): Oral: 400 mg twice daily (Ottmann 2013)
Chronic myeloid leukemia (CML), Ph+, newly-diagnosed in chronic phase: Oral: 300 mg twice daily (Kantarjian 2011b; Saglio 2010)
Discontinuation of therapy (following a sustained molecular response [MR4.5]): May consider nilotinib discontinuation in patients who have been treated with nilotinib for at least 3 years, maintained a molecular response of at least MR4.0 (corresponding to BCR-ABL/ABL ≤0.01% IS) for 1 year (prior to discontinuation), achieved an MR4.5 (corresponding to BCR-ABL/ABL ≤0.0032% IS) for the last assessment taken immediately prior to discontinuation, been confirmed to express the typical BCR-ABL transcripts (e13a2/b2a2 or e14a2/b3a2), no history of accelerated phase or blast crisis, and no history of prior attempts of treatment-free remission discontinuation that resulted in relapse.
Reinitiation of therapy: Patients who lose major molecular response after nilotinib discontinuation must reinitiate treatment within 4 weeks at the dose used prior to discontinuation. Monitor BCR-ABL transcript levels monthly until major molecular response is re-established and every 12 weeks thereafter. BCR-ABL kinase domain mutation testing should be performed if major molecular response is not achieved after 3 months of therapy reinitiation.
CML, Ph+, resistant or intolerant in accelerated phase: Oral: 400 mg twice daily (le Coutre 2012)
CML, Ph+, resistant or intolerant in chronic phase: Oral: 400 mg twice daily (Kantarjian 2007; Kantarjian 2011a)
Discontinuation of therapy (following a sustained molecular response [MR4.5]): May consider nilotinib discontinuation in patients with CML in chronic phase (resistant or intolerant to prior imatinib therapy) who have been treated with nilotinib for at least 3 years, been treated with imatinib (only) prior to nilotinib treatment, achieved an MR4.5 (corresponding to BCR-ABL/ABL ≤0.0032% IS), achieved a sustained MR4.5 for a minimum of 1 year immediately prior to discontinuation, been confirmed to express the typical BCR-ABL transcripts (e13a2/b2a2 or e14a2/b3a2), no history of accelerated phase or blast crisis, and no history of prior attempts of treatment-free remission discontinuation that resulted in relapse.
Reinitiation of therapy: Patients with confirmed loss of MR4.0 (2 consecutive readings separated by at least 4 weeks showing loss of MR4.0) or loss of major molecular response after nilotinib discontinuation must reinitiate treatment within 4 weeks at the dose used prior to discontinuation. Monitor BCR-ABL transcript levels monthly until previous major molecular response or MR4.0 is re-established and every 12 weeks thereafter. BCR-ABL kinase domain mutation testing should be performed if major molecular response is not achieved after 3 months of therapy reinitiation.
Gastrointestinal stromal tumor (GIST), refractory (off-label use): Oral: 400 mg twice daily until disease progression or unacceptable toxicity (Reichardt 2012)
Missed doses: If a dose is missed, do not make up, resume with next scheduled dose.
Dosage adjustment for concomitant CYP3A4 inhibitors/inducers:
CYP3A4 inhibitors: Avoid the concomitant use of a strong CYP3A4 inhibitor with nilotinib. If a strong CYP3A4 inhibitor is required, interrupt nilotinib treatment.
If therapy cannot be interrupted and concurrent use with a strong CYP3A4 inhibitor cannot be avoided, reduce the nilotinib dose to 300 mg once daily in patients with resistant or intolerant Ph+ CML (chronic or accelerated phase) or to 200 mg once daily in newly diagnosed chronic phase Ph+ CML, with careful monitoring, especially of the QT interval. When a strong CYP3A4 inhibitor is discontinued, allow a washout period prior to adjusting nilotinib dose upward.
CYP3A4 inducers: Avoid the concomitant use of a strong CYP3A4 inducer with nilotinib (based on pharmacokinetic parameters, an increased nilotinib dose is not likely to compensate for decreased exposure).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Chronic myeloid leukemia (CML), Philadelphia chromosome-positive (Ph+), newly diagnosed in chronic phase: Children and Adolescents: Oral: 230 mg/m2/dose twice daily; round dose to the nearest 50 mg increment; maximum dose: 400 mg/dose. Continue therapy as long as observing clinical benefit or until unacceptable toxicity occurs.
CML, Ph+, resistant or intolerant in chronic phase: Children and Adolescents: Oral: 230 mg/m2/dose twice daily; round dose to the nearest 50 mg increment; maximum dose: 400 mg/dose. Continue therapy as long as observing clinical benefit or until unacceptable toxicity occurs.
Dosing adjustment for concomitant CYP3A4 inhibitors/inducers: Children and Adolescents:
CYP3A4 inhibitors: Avoid the concomitant use of a strong CYP3A4 inhibitor with nilotinib. If a strong CYP3A4 inhibitor is required, interrupt nilotinib treatment.
If therapy cannot be interrupted and concurrent use with a strong CYP3A4 inhibitor cannot be avoided, experience in adult patients suggests that reduced dosing is necessary; there are no pediatric-specific recommendations. Patients should be closely monitored; especially ECG (QT interval). When a strong CYP3A4 inhibitor is discontinued, allow a washout period prior to adjusting nilotinib dose upward.
CYP3A4 inducers: Avoid the concomitant use of a strong CYP3A4 inducer with nilotinib (based on pharmacokinetic parameters, an increased nilotinib dose is not likely to compensate for decreased exposure).
Dosage adjustment toxicity unrelated to underlying leukemia: Children and Adolescents: Oral:
Hematologic toxicity (unrelated to underlying leukemia):
ANC <1,000/mm3 and/or platelets <50,000/mm3: Withhold treatment, monitor blood counts
If ANC >1,500/mm3 and/or platelets >75,000/mm3 within 2 weeks: Resume at prior dose
If blood counts remain low for >2 weeks: May need to reduce dose to 230 mg/m2 once daily
If toxicity occurs after dose reduction, consider discontinuing treatment.
Nonhematologic toxicity:
Amylase or lipase elevation ≥ grade 3: Withhold treatment, monitor serum amylase or lipase. When lipase or amylase returns to ≤ grade 1, resume treatment at 230 mg/m2 once daily (if previous dose was 230 mg/m2 twice daily). If previous dose was 230 mg/m2 once daily, discontinue therapy.
Lipase increases in conjunction with abdominal symptoms: Withhold treatment and consider diagnostics to exclude pancreatitis
Clinically significant moderate or severe nonhematologic toxicity (including medically severe fluid retention): Withhold treatment; upon resolution of toxicity, resume at 230 mg/m2 once daily (if previous dose was 230 mg/m2 twice daily); may escalate back to initial dose (230 mg/m2 twice daily) if clinically appropriate. Discontinue if previous dose was 230 mg/m2 once daily.
QT prolongation: Note: Repeat ECG ~7 days after any dosage adjustment
QTc >480 msec: Withhold treatment, monitor serum potassium and magnesium levels and correct if necessary; review concurrent medications
If QTcF returns to <450 msec and to within 20 msec of baseline within 2 weeks: Resume at prior dose
If QTcF returns to 450 to 480 msec after 2 weeks: Reduce dose to 230 mg/m2 once daily; repeat ECG in approximately 7 days
If QTcF >480 msec after dosage reduction to 230 mg/m2 once daily: Discontinue treatment
Dosing: Adjustment for Toxicity
Chronic myeloid leukemia (Philadelphia chromosome-positive [Ph+]):
Dosage adjustment for hematologic toxicity unrelated to underlying leukemia:
ANC <1,000/mm3 and/or platelets <50,000/mm3: Withhold treatment, monitor blood counts
If ANC >1,000/mm3 and platelets >50,000/mm3 within 2 weeks: Resume at prior dose
If blood counts remain low for >2 weeks: Reduce dose to 400 mg once daily
Dosage adjustment for nonhematologic toxicity:
Amylase or lipase ≥ grade 3: Withhold treatment, monitor serum amylase or lipase, resume treatment at 400 mg once daily when lipase or amylase returns to ≤ grade 1
Lipase increases in conjunction with abdominal symptoms: Withhold treatment and consider diagnostics to exclude pancreatitis
Clinically significant moderate or severe nonhematologic toxicity (including medically severe fluid retention): Withhold treatment, upon resolution of toxicity, resume at 400 mg once daily (if previous dose was 300 mg twice daily or 400 mg twice daily, depending on indication); may escalate back to initial dose (300 mg twice daily or 400 mg twice daily, depending on indication) if clinically appropriate. Discontinue if previous dose was 400 mg once daily.
Dosage adjustment for QT prolongation: Note: Repeat ECG ~7 days after any dosage adjustment
QTc >480 msec: Withhold treatment, monitor and correct potassium and magnesium levels; review concurrent medications
If QTcF returns to <450 msec and to within 20 msec of baseline within 2 weeks: Resume at prior dose
If QTcF returns to 450 to 480 msec after 2 weeks: Reduce dose to 400 mg once daily (adults)
If QTcF >480 msec after dosage reduction to 400 mg once daily (adults): Discontinue treatment
Acute lymphoblastic leukemia, Ph+, newly diagnosed (off-label use): Temporarily reduce nilotinib dose to 400 mg once daily for ≥ grade 3 nonhematologic toxicity (resume when improved to grade 1); severe recurrent adverse events despite dose reduction may require permanent discontinuation (Kim 2015)
Administration
Oral: Administer twice daily with doses ~12 hours apart. Administer on an empty stomach, at least 1 hour before or 2 hours after food. Note: The prescribing information describes when to give food with respect to nilotinib; no food should be consumed for at least 2 hours before or for at least 1 hour after the nilotinib dose. Capsules should be swallowed whole with water. If unable to swallow whole, may empty contents into 5 mL applesauce (puréed apple) and administer within 15 minutes (do not save for later use).
Dietary Considerations
The bioavailability of nilotinib is increased with food.
Storage
Store at 25°C (77°F); excursions are permitted between 15°C and 30°C (59°F and 86°F).
Nilotinib Images
Drug Interactions
Abemaciclib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Abemaciclib. Monitor therapy
Acalabrutinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Acalabrutinib. Management: Reduce acalabrutinib dose to 100 mg once daily with concurrent use of a moderate CYP3A4 inhibitor. Monitor patient closely for both acalabrutinib response and evidence of adverse effects with any concurrent use. Consider therapy modification
AmLODIPine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of AmLODIPine. Monitor therapy
Antacids: May decrease the serum concentration of Nilotinib. Management: Separate the administration of nilotinib and any antacid by at least 2 hours whenever possible in order to minimize the risk of a significant interaction. Consider therapy modification
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Apixaban: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Apixaban. Monitor therapy
Aprepitant: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Aprepitant. Avoid combination
ARIPiprazole: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of ARIPiprazole. Management: Monitor for increased aripiprazole pharmacologic effects. Aripiprazole dose adjustments may or may not be required based on concomitant therapy and/or indication. Consult full interaction monograph for specific recommendations. Monitor therapy
Asunaprevir: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Asunaprevir. Avoid combination
Avanafil: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Avanafil. Management: The maximum avanafil adult dose is 50 mg per 24-hour period when used together with a moderate CYP3A4 inhibitor. Patients receiving such a combination should also be monitored more closely for evidence of adverse effects. Consider therapy modification
Avapritinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Avapritinib. Management: Avoid use of moderate CYP3A4 inhibitors with avapritinib. If this combination cannot be avoided, reduce the avapritinib dose from 300 mg once daily to 100 mg once daily. Consider therapy modification
Axitinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Axitinib. Monitor therapy
Baricitinib: Immunosuppressants may enhance the immunosuppressive effect of Baricitinib. Management: Use of baricitinib in combination with potent immunosuppressants such as azathioprine or cyclosporine is not recommended. Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted. Consider therapy modification
BCG (Intravesical): Immunosuppressants may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
BCG (Intravesical): Myelosuppressive Agents may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Benzhydrocodone: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Benzhydrocodone. Specifically, the concentration of hydrocodone may be increased. Monitor therapy
Blonanserin: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Blonanserin. Monitor therapy
Bosentan: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Bosentan: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Bosentan. Management: Concomitant use of both a CYP2C9 inhibitor and a CYP3A inhibitor or a single agent that inhibits both enzymes with bosentan is likely to cause a large increase in serum concentrations of bosentan and is not recommended. See monograph for details. Monitor therapy
Bosutinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Bosutinib. Avoid combination
Brexpiprazole: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Brexpiprazole. Management: The brexpiprazole dose should be reduced to 25% of usual if used together with both a moderate CYP3A4 inhibitor and a strong or moderate CYP2D6 inhibitor, or if a moderate CYP3A4 inhibitor is used in a CYP2D6 poor metabolizer. Monitor therapy
Brigatinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Brigatinib. Management: Avoid concurrent use of brigatinib with moderate CYP3A4 inhibitors when possible. If such a combination cannot be avoided, reduce the dose of brigatinib by approximately 40% (ie, from 180 mg to 120 mg, from 120 mg to 90 mg, or from 90 mg to 60 mg). Consider therapy modification
Bromocriptine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Bromocriptine. Management: The bromocriptine dose should not exceed 1.6 mg daily with use of a moderate CYP3A4 inhibitor. The Cycloset brand specifically recommends this dose limitation, but other bromocriptine products do not make such specific recommendations. Consider therapy modification
Budesonide (Systemic): CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Budesonide (Systemic). Avoid combination
Budesonide (Topical): CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Budesonide (Topical). Management: Per US prescribing information, avoid this combination. Canadian product labeling does not recommend strict avoidance. If combined, monitor for excessive glucocorticoid effects as budesonide exposure may be increased. Consider therapy modification
Cannabidiol: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Cannabidiol. Monitor therapy
Cannabis: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Cannabis. More specifically, tetrahydrocannabinol and cannabidiol serum concentrations may be increased. Monitor therapy
Chloramphenicol (Ophthalmic): May enhance the adverse/toxic effect of Myelosuppressive Agents. Monitor therapy
Cilostazol: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Cilostazol. Management: Consider reducing the cilostazol dose to 50 mg twice daily in adult patients who are also receiving moderate inhibitors of CYP3A4. Consider therapy modification
Citalopram: May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of Citalopram. Monitor therapy
Cladribine: May enhance the immunosuppressive effect of Immunosuppressants. Avoid combination
Cladribine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Avoid combination
Clofazimine: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Cobimetinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Cobimetinib. Management: Avoid the concomitant use of cobimetinib and moderate CYP3A4 inhibitors. If concurrent short term (14 days or less) use cannot be avoided, reduce the cobimetinib dose to 20 mg daily. Avoid combination
Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Monitor therapy
Codeine: CYP3A4 Inhibitors (Moderate) may increase serum concentrations of the active metabolite(s) of Codeine. Monitor therapy
Colchicine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Colchicine. Management: Reduce colchicine dose as directed when using with a moderate CYP3A4 inhibitor, and increase monitoring for colchicine-related toxicity. See full monograph for details. Use extra caution in patients with impaired renal and/or hepatic function. Consider therapy modification
Conivaptan: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Crizotinib: QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may enhance the QTc-prolonging effect of Crizotinib. QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of Crizotinib. Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May decrease the serum concentration of Nilotinib. Avoid combination
CYP3A4 Inhibitors (Moderate): May decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CYP3A4 Inhibitors (Strong): May increase the serum concentration of Nilotinib. Management: Avoid if possible. If combination needed, decrease nilotinib to 300 mg once/day for patients with resistant or intolerant Ph+ CML or to 200 mg once/day for patients with newly diagnosed Ph+ CML in chronic phase. Exceptions discussed in separate monograph. Exceptions: Ceritinib; Clarithromycin; Saquinavir; Voriconazole. Consider therapy modification
CYP3A4 Substrates (High risk with Inhibitors): CYP3A4 Inhibitors (Moderate) may decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Exceptions: Alitretinoin (Systemic); Praziquantel; Trabectedin; Vinorelbine. Monitor therapy
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Dapoxetine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Dapoxetine. Management: The dose of dapoxetine should be limited to 30 mg per day when used together with a moderate inhibitor of CYP3A4. Consider therapy modification
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Deferiprone: Myelosuppressive Agents may enhance the neutropenic effect of Deferiprone. Management: Avoid the concomitant use of deferiprone and myelosuppressive agents whenever possible. If this combination cannot be avoided, monitor the absolute neutrophil count more closely. Consider therapy modification
Deflazacort: CYP3A4 Inhibitors (Moderate) may increase serum concentrations of the active metabolite(s) of Deflazacort. Management: Administer one third of the recommended deflazacort dose when used together with a strong or moderate CYP3A4 inhibitor. Consider therapy modification
Denosumab: May enhance the adverse/toxic effect of Immunosuppressants. Specifically, the risk for serious infections may be increased. Monitor therapy
Dipyrone: May enhance the adverse/toxic effect of Myelosuppressive Agents. Specifically, the risk for agranulocytosis and pancytopenia may be increased Avoid combination
Domperidone: May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of Domperidone. Avoid combination
DOXOrubicin (Conventional): CYP3A4 Inhibitors (Moderate) may increase the serum concentration of DOXOrubicin (Conventional). Management: Seek alternatives to moderate CYP3A4 inhibitors in patients treated with doxorubicin whenever possible. One U.S. manufacturer (Pfizer Inc.) recommends that these combinations be avoided. Consider therapy modification
Dronabinol: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Dronabinol. Monitor therapy
Duvelisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Echinacea: May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification
Eletriptan: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Eletriptan. Management: The use of eletriptan within 72 hours of a moderate CYP3A4 inhibitor should be avoided. Consider therapy modification
Elexacaftor, Tezacaftor, and Ivacaftor: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Elexacaftor, Tezacaftor, and Ivacaftor. Management: When combined with moderate CYP3A4 inhibitors, two elexacaftor/tezacaftor/ivacaftor (100 mg/50 mg/75 mg) tablets should be given in the morning, every other day. Ivacaftor (150 mg) should be given in the morning, every other day on alternate days. Consider therapy modification
Eliglustat: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Eliglustat. Management: Use should be avoided under some circumstances. See full drug interaction monograph for details. Consider therapy modification
Encorafenib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Encorafenib. Management: Avoid concomitant use of encorafenib and moderate CYP3A4 inhibitors whenever possible. If concomitant administration is unavoidable, decrease the encorafenib dose prior to initiation of the CYP3A4 inhibitor. See full monograph for details. Consider therapy modification
Encorafenib: May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of Encorafenib. Management: Avoid using moderate CYP3A4 inhibitors together with encorafenib if possible. If the combination must be used, reduce the encorafenib dose prior to initiation of the moderate CYP3A4 inhibitor and monitor QT interval. See full monograph for details. Consider therapy modification
Entrectinib: May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of Entrectinib. Avoid combination
Eplerenone: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Eplerenone. Management: When used concomitantly with moderate inhibitors of CYP3A4, eplerenone dosing recommendations vary by indication and international labeling. See full drug interaction monograph for details. Consider therapy modification
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Erdafitinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Erythromycin (Systemic): May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Estrogen Derivatives: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Estrogen Derivatives. Monitor therapy
Everolimus: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Everolimus. Management: Everolimus dose reductions are required for most indications. See full monograph or prescribing information for specific dose adjustment and monitoring recommendations. Consider therapy modification
FentaNYL: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of FentaNYL. Management: Monitor patients closely for several days following initiation of this combination, and adjust fentanyl dose as necessary. Consider therapy modification
Fexinidazole [INT]: May enhance the QTc-prolonging effect of QT-prolonging Agents (Moderate Risk). Avoid combination
Fingolimod: Immunosuppressants may enhance the immunosuppressive effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and other immunosuppressants when possible. If combined, monitor patients closely for additive immunosuppressant effects (eg, infections). Consider therapy modification
Flibanserin: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Flibanserin. Avoid combination
Fluconazole: May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Fosaprepitant: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Fosaprepitant. Avoid combination
Fosnetupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fusidic Acid (Systemic): May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Grapefruit Juice: May increase the serum concentration of Nilotinib. Monitor therapy
GuanFACINE: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of GuanFACINE. Management: Reduce the guanfacine dose by 50% when initiating this combination. Consider therapy modification
Haloperidol: QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may enhance the QTc-prolonging effect of Haloperidol. Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Histamine H2 Receptor Antagonists: May decrease the serum concentration of Nilotinib. Management: The nilotinib dose should be given 10 hours after or 2 hours before the H2 receptor antagonist in order to minimize the risk of a significant interaction. Consider therapy modification
HYDROcodone: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of HYDROcodone. Monitor therapy
Ibrutinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Ibrutinib. Management: When treating B-cell malignancies, decrease ibrutinib to 280 mg daily when combined with moderate CYP3A4 inhibitors. When treating graft versus host disease, monitor patients closely and reduce the ibrutinib dose as needed based on adverse reactions. Consider therapy modification
Idelalisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Ifosfamide: CYP3A4 Inhibitors (Moderate) may decrease serum concentrations of the active metabolite(s) of Ifosfamide. Monitor therapy
Imatinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Imatinib. Monitor therapy
Ivabradine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Ivabradine. Avoid combination
Ivacaftor: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Ivacaftor. Management: Ivacaftor dose reductions may be required; consult full monograph content for age- and weight-specific dosage recommendations. Consider therapy modification
Ivosidenib: Nilotinib may enhance the QTc-prolonging effect of Ivosidenib. Nilotinib may increase the serum concentration of Ivosidenib. Avoid combination
Larotrectinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Lefamulin: May enhance the QTc-prolonging effect of QT-prolonging CYP3A4 Substrates. Management: Do not use lefamulin tablets with QT-prolonging CYP3A4 substrates. Lefamulin prescribing information lists this combination as contraindicated. Avoid combination
Leflunomide: Immunosuppressants may enhance the adverse/toxic effect of Leflunomide. Specifically, the risk for hematologic toxicity such as pancytopenia, agranulocytosis, and/or thrombocytopenia may be increased. Management: Consider not using a leflunomide loading dose in patients receiving other immunosuppressants. Patients receiving both leflunomide and another immunosuppressant should be monitored for bone marrow suppression at least monthly. Consider therapy modification
Lemborexant: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Lemborexant. Avoid combination
Levamlodipine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Levamlodipine. Monitor therapy
Lomitapide: Nilotinib may increase the serum concentration of Lomitapide. Management: Patients taking lomitapide 10 mg/day or more should decrease the lomitapide dose by half with concurrent nilotinib; the lomitapide dose may then be increased to a max adult dose of 30 mg/day (patients on lomitapide 5 mg/day may continue that dose). Consider therapy modification
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
Lumateperone: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Lumateperone. Avoid combination
Lurasidone: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Lurasidone. Management: Lurasidone US labeling recommends reducing lurasidone dose by half with a moderate CYP3A4 inhibitor. Some non-US labeling recommends initiating lurasidone at 20 mg/day and limiting dose to 40 mg/day; avoid concurrent use of grapefruit products. Consider therapy modification
Manidipine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Manidipine. Monitor therapy
Meperidine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Meperidine. Monitor therapy
Mesalamine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
MiFEPRIStone: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Minimize doses of CYP3A4 substrates, and monitor for increased concentrations/toxicity, during and 2 weeks following treatment with mifepristone. Avoid cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, and tacrolimus. Consider therapy modification
Mirodenafil: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Mirodenafil. Monitor therapy
Naldemedine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Naldemedine. Monitor therapy
Nalfurafine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Nalfurafine. Monitor therapy
Naloxegol: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Naloxegol. Avoid combination
Natalizumab: Immunosuppressants may enhance the adverse/toxic effect of Natalizumab. Specifically, the risk of concurrent infection may be increased. Avoid combination
Neratinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Neratinib. Avoid combination
Netupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
NiMODipine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of NiMODipine. Monitor therapy
Nivolumab: Immunosuppressants may diminish the therapeutic effect of Nivolumab. Consider therapy modification
Ocrelizumab: May enhance the immunosuppressive effect of Immunosuppressants. Monitor therapy
Olaparib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Olaparib. Management: Avoid use of moderate CYP3A4 inhibitors in patients being treated with olaparib, if possible. If such concurrent use cannot be avoided, the dose of olaparib should be reduced to 150 mg twice daily. Consider therapy modification
Ondansetron: May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
OxyCODONE: CYP3A4 Inhibitors (Moderate) may enhance the adverse/toxic effect of OxyCODONE. CYP3A4 Inhibitors (Moderate) may increase the serum concentration of OxyCODONE. Serum concentrations of the active metabolite Oxymorphone may also be increased. Monitor therapy
Palbociclib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Pentamidine (Systemic): May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Pexidartinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Pexidartinib. Monitor therapy
Pidotimod: Immunosuppressants may diminish the therapeutic effect of Pidotimod. Monitor therapy
Pimecrolimus: May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Pimozide: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Pimozide. Avoid combination
Pimozide: May enhance the QTc-prolonging effect of QT-prolonging Agents (Moderate Risk). Avoid combination
Posaconazole: May increase the serum concentration of QT-prolonging CYP3A4 Substrates. Such increases may lead to a greater risk for proarrhythmic effects and other similar toxicities. Avoid combination
Promazine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Proton Pump Inhibitors: May decrease the serum concentration of Nilotinib. Management: Avoid this combination when possible since separation of doses is not likely to be an adequate method of minimizing the interaction. Consider therapy modification
QT-prolonging Agents (Highest Risk): May enhance the QTc-prolonging effect of Nilotinib. Exceptions: Ivosidenib. Avoid combination
QT-prolonging Antidepressants (Moderate Risk): May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). Exceptions: Citalopram. Monitor therapy
QT-prolonging Antipsychotics (Moderate Risk): QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may enhance the QTc-prolonging effect of QT-prolonging Antipsychotics (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Exceptions: Pimozide; QUEtiapine. Monitor therapy
QT-prolonging Class IC Antiarrhythmics (Moderate Risk): May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
QT-prolonging Kinase Inhibitors (Moderate Risk): QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may enhance the QTc-prolonging effect of QT-prolonging Kinase Inhibitors (Moderate Risk). QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of QT-prolonging Kinase Inhibitors (Moderate Risk). Exceptions: Encorafenib; Entrectinib. Monitor therapy
QT-prolonging Miscellaneous Agents (Moderate Risk): QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may enhance the QTc-prolonging effect of QT-prolonging Miscellaneous Agents (Moderate Risk). QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of QT-prolonging Miscellaneous Agents (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Exceptions: Domperidone. Monitor therapy
QT-prolonging Quinolone Antibiotics (Moderate Risk): May enhance the QTc-prolonging effect of QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
QT-prolonging Strong CYP3A4 Inhibitors (Moderate Risk): Nilotinib may enhance the QTc-prolonging effect of QT-prolonging Strong CYP3A4 Inhibitors (Moderate Risk). QT-prolonging Strong CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of Nilotinib. Management: Avoid concomitant use of nilotinib and strong CYP3A4 inhibitors that prolong the QTc interval whenever possible. If combined, nilotinib dose reductions are required. Monitor patients for nilotinib toxicities including QTc prolongation and arrhythmias. Consider therapy modification
QUEtiapine: QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may enhance the QTc-prolonging effect of QUEtiapine. QT-prolonging Moderate CYP3A4 Inhibitors (Moderate Risk) may increase the serum concentration of QUEtiapine. Management: Monitor for increased quetiapine toxicities including QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Ranolazine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Ranolazine. Management: Limit the ranolazine adult dose to a maximum of 500 mg twice daily in patients concurrently receiving moderate CYP3A4 inhibitors (e.g., diltiazem, verapamil, erythromycin, etc.). Consider therapy modification
Ribociclib: Nilotinib may enhance the QTc-prolonging effect of Ribociclib. Management: Consider alternatives to this combination. Patients with other risk factors (eg, older age, female sex, bradycardia, hypokalemia, hypomagnesemia, heart disease, and higher drug concentrations) are likely at greater risk for these toxicities. Consider therapy modification
Rifabutin: May decrease the serum concentration of Nilotinib. Monitor therapy
Rifapentine: May decrease the serum concentration of Nilotinib. Monitor therapy
Roflumilast: May enhance the immunosuppressive effect of Immunosuppressants. Consider therapy modification
Rupatadine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Rupatadine. Monitor therapy
Ruxolitinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Ruxolitinib. Monitor therapy
Salmeterol: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Salmeterol. Monitor therapy
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
SAXagliptin: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of SAXagliptin. Monitor therapy
Sildenafil: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Sildenafil. Monitor therapy
Silodosin: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Silodosin. Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Simeprevir: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Simeprevir. Avoid combination
Siponimod: Immunosuppressants may enhance the immunosuppressive effect of Siponimod. Monitor therapy
Sipuleucel-T: Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Management: Evaluate patients to see if it is medically appropriate to reduce or discontinue therapy with immunosuppressants prior to initiating sipuleucel-T therapy. Consider therapy modification
Sirolimus: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Sirolimus. Management: Monitor for increased serum concentrations of sirolimus if combined with a moderate CYP3A4 inhibitor. Lower initial sirolimus doses or sirolimus dose reductions will likely be required. Consider therapy modification
Smallpox and Monkeypox Vaccine (Live): Immunosuppressants may diminish the therapeutic effect of Smallpox and Monkeypox Vaccine (Live). Monitor therapy
Sonidegib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Sonidegib. Management: Avoid concomitant use of sonidegib and moderate CYP3A4 inhibitors when possible. When concomitant use cannot be avoided, limit CYP3A4 inhibitor use to less than 14 days and monitor for sonidegib toxicity (particularly musculoskeletal adverse reactions). Consider therapy modification
St John's Wort: May decrease the serum concentration of Nilotinib. Avoid combination
Stiripentol: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Use of stiripentol with CYP3A4 substrates that are considered to have a narrow therapeutic index should be avoided due to the increased risk for adverse effects and toxicity. Any CYP3A4 substrate used with stiripentol requires closer monitoring. Consider therapy modification
Suvorexant: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Suvorexant. Management: The recommended dose of suvorexant is 5 mg daily in patients receiving a moderate CYP3A4 inhibitor. The dose can be increased to 10 mg daily (maximum dose) if necessary for efficacy. Consider therapy modification
Tacrolimus (Topical): May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Tamsulosin: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Tamsulosin. Monitor therapy
Tazemetostat: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Tazemetostat. Management: Avoid coadministration of tazemetostat and moderate CYP3A4 inhibitors. If coadministration cannot be avoided, dose reductions are required. See full monograph for dosing recommendations. Consider therapy modification
Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide. Monitor therapy
Tetrahydrocannabinol: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Tetrahydrocannabinol. Monitor therapy
Tezacaftor and Ivacaftor: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Tezacaftor and Ivacaftor. Management: When combined with moderate CYP3A4 inhibitors, tezacaftor/ivacaftor should be given in the morning, every other day. Ivacaftor alone should be given in the morning, every other day on alternate days. Consider therapy modification
Ticagrelor: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Ticagrelor. Monitor therapy
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Tofacitinib: Immunosuppressants may enhance the immunosuppressive effect of Tofacitinib. Management: Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted, and this warning seems particularly focused on more potent immunosuppressants. Consider therapy modification
Tolvaptan: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Tolvaptan. Management: Jynarque dose requires adjustment when used with a moderate CYP3A4 inhibitor. See labeling or full interaction monograph for specific recommendations. Use of Samsca with moderate CYP3A4 ihibitors should generally be avoided. Consider therapy modification
Trabectedin: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Trabectedin. Monitor therapy
Trastuzumab: May enhance the neutropenic effect of Immunosuppressants. Monitor therapy
Triazolam: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Triazolam. Management: Consider triazolam dose reduction in patients receiving concomitant moderate CYP3A4 inhibitors. Consider therapy modification
Ubrogepant: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Ubrogepant. Management: Use an initial ubrogepant dose of 50 mg and avoid a second dose for 24 hours when used with moderate CYP3A4 inhibitors. Consider therapy modification
Udenafil: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Udenafil. Monitor therapy
Ulipristal: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Ulipristal. Management: This is specific for when ulipristal is being used for signs/symptoms of uterine fibroids (Canadian indication). When ulipristal is used as an emergency contraceptive, patients receiving this combination should be monitored for ulipristal toxicity. Avoid combination
Upadacitinib: Immunosuppressants may enhance the immunosuppressive effect of Upadacitinib. Avoid combination
Vaccines (Inactivated): Immunosuppressants may diminish the therapeutic effect of Vaccines (Inactivated). Management: Vaccine efficacy may be reduced. Complete all age-appropriate vaccinations at least 2 weeks prior to starting an immunosuppressant. If vaccinated during immunosuppressant therapy, revaccinate at least 3 months after immunosuppressant discontinuation. Consider therapy modification
Vaccines (Live): Immunosuppressants may enhance the adverse/toxic effect of Vaccines (Live). Immunosuppressants may diminish the therapeutic effect of Vaccines (Live). Management: Avoid use of live organism vaccines with immunosuppressants; live-attenuated vaccines should not be given for at least 3 months after immunosuppressants. Exceptions: Smallpox and Monkeypox Vaccine (Live). Avoid combination
Venetoclax: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Venetoclax. Management: Reduce the venetoclax dose by at least 50% in patients requiring these combinations. Consider therapy modification
Vilazodone: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Vilazodone. Monitor therapy
Vindesine: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Vindesine. Monitor therapy
Zanubrutinib: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Zanubrutinib. Management: Decrease the zanubrutinib dose to 80 mg twice daily during coadministration with a moderate CYP3A4 inhibitor. Further dose adjustments may be required for zanubrutinib toxicities, refer to prescribing information for details. Consider therapy modification
Zopiclone: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Zopiclone. Management: The starting adult dose of zopiclone should not exceed 3.75 mg if combined with a moderate CYP3A4 inhibitor. Monitor patients for signs and symptoms of zopiclone toxicity if these agents are combined. Consider therapy modification
Zuclopenthixol: CYP3A4 Inhibitors (Moderate) may increase the serum concentration of Zuclopenthixol. Monitor therapy
Adverse Reactions
>10%:
Cardiovascular: Prolonged QT interval on ECG (children and adolescents: >30 msec from baseline: 25%; adults: >60 msec from baseline: 4%; adults: >500 msec: <1%), occlusive arterial disease (9% to 15%), peripheral edema (9% to 15%), hypertension (10% to 11%)
Central nervous system: Headache (20% to 35%; children & adolescents: >20%), fatigue (23% to 32%), dizziness (12%), insomnia (7% to 12%)
Dermatologic: Skin rash (29% to 38%; children & adolescents: >20%), pruritus (20% to 32%), night sweats (12% to 27%), alopecia (11% to 13%), xeroderma (12%)
Endocrine & metabolic: Hyperglycemia (≤50%), hypophosphatemia (grade 3/4: 8% to 17%), increased HDL cholesterol, increased VLDL
Gastrointestinal: Nausea (22% to 37%; children & adolescents: >20%), vomiting (13% to 29%; children & adolescents: >20%), increased serum lipase (28%), diarrhea (19% to 28%), constipation (19% to 26%), upper abdominal pain (12% to 18%), decreased appetite (including anorexia; 15% to 17%), abdominal pain (15% to 16%)
Hematologic & oncologic: Neutropenia (15%; grades 3/4: 12% to 42%; children & adolescents: 41%), thrombocytopenia (18%; grades 3/4: 10% to 42%; children & adolescents: 44%), decreased absolute lymphocyte count (children & adolescents: 32%), anemia (8%; grades 3/4: 4% to 27%; children & adolescents: 30%)
Hepatic: Increased serum alanine aminotransferase (10% to 72%; children & adolescents: >20%), increased serum aspartate aminotransferase (10% to 47%; children & adolescents: >20%), hyperbilirubinemia (≥10%; children & adolescents: >20%)
Infection: Influenza (13%)
Neuromuscular & skeletal: Arthralgia (16% to 26%), limb pain (15% to 20%), myalgia (16% to 19%), back pain (15% to 19%), asthenia (14% to 16%), ostealgia (14% to 15%), muscle spasm (12% to 15%), musculoskeletal pain (11% to 12%)
Respiratory: Cough (17% to 27%), nasopharyngitis (15% to 27%), upper respiratory tract infection (10% to 17%; children & adolescents: >20%), dyspnea (9% to 15%), oropharyngeal pain (7% to 12%)
Miscellaneous: Fever (14% to 28%; children & adolescents: >20%)
1% to 10%:
Cardiovascular: Ischemic heart disease (≤9%), peripheral arterial disease (≤4%), cerebral ischemia (1% to 3%), pericardial effusion (≤2%), angina pectoris, cardiac arrhythmia (including AV block, atrial fibrillation, bradycardia, cardiac flutter, extrasystoles, and tachycardia), chest discomfort, chest pain (including noncardiac), flushing, palpitations
Central nervous system: Anxiety, depression, flank pain, hypoesthesia, malaise, myasthenia, pain, paresthesia, peripheral neuropathy, vertigo, voice disorder
Dermatologic: Acne vulgaris, dermatitis (including allergic, exfoliative, and acneiform), eczema, erythema of skin, folliculitis, hyperhidrosis, urticaria
Endocrine & metabolic: Decreased serum albumin (grade 3/4: 3% to 4%), fluid retention (grade 3/4: 3% to 4%), altered hormone level (growth hormone deficiency; children and adolescents: 2%), diabetes mellitus, electrolyte disorder, hypercalcemia, hypercholesterolemia, hyperkalemia, hyperlipidemia, hyperphosphatemia, hypertriglyceridemia, hypocalcemia, hypokalemia, hypomagnesemia, hyponatremia, increased gamma-glutamyl transferase, weight gain, weight loss
Gastrointestinal: Dyspepsia (4% to 10%), gastroenteritis (7%), gastrointestinal hemorrhage (≤5%), abdominal distension, abdominal distress, dysgeusia, flatulence, increased serum amylase, pancreatitis
Genitourinary: Pollakiuria
Hematologic & oncologic: Hemorrhage (grade 3/4: 1% to 2%), bruise, change in serum protein (decreased globulins), cutaneous papilloma, eosinophilia, febrile neutropenia, hemophthalmos, leukopenia, lymphocytopenia, pancytopenia
Hepatic: Ascites (≤2%), hepatic insufficiency, increased serum alkaline phosphatase
Neuromuscular & skeletal: Increased creatine phosphokinase in blood specimen, musculoskeletal chest pain, neck pain
Ophthalmic: Eyelid edema (1%), conjunctivitis, eye pruritus, xerophthalmia
Respiratory: Pleural effusion (≤2%), dyspnea on exertion, epistaxis
Frequency not defined:
Cardiovascular: Hypotension, pericarditis, reduced ejection fraction, shock (hemorrhagic), thrombosis, ventricular dysfunction
Central nervous system: Amnesia, breast induration, cerebral edema, confusion, disorientation, dysesthesia, dysphoria, lethargy, restless leg syndrome
Dermatologic: Cutaneous nodule (sebaceous hyperplasia), dermal ulcer, erythema multiforme, erythema nodosum, exfoliation of skin, furuncle, hyperkeratosis, palmar-plantar erythrodysesthesia, psoriasis, skin atrophy, skin blister, skin discoloration, skin hyperpigmentation, skin hypertrophy, skin photosensitivity, tinea pedis
Endocrine & metabolic: Altered hormone level (insulin C-peptide decreased), growth retardation, hyperparathyroidism (secondary), hyperuricemia, hypoglycemia, thyroiditis
Gastrointestinal: Cholestasis, enterocolitis, gastric ulcer (perforation possible), gingivitis, hematemesis, hemorrhoids, hiatal hernia, intestinal obstruction, oral lesion (papilloma), ulcerative esophagitis
Genitourinary: Dysmenorrhea, hematuria, urinary incontinence, urine discoloration
Hematologic & oncologic: Leukocytosis, paraproteinemia, petechia, rectal hemorrhage, retroperitoneal hemorrhage, thrombocythemia
Hepatic: Hepatomegaly
Hypersensitivity: Hypersensitivity
Infection: Abscess (subcutaneous), anal abscess, reactivation of HBV, sepsis
Local: Local swelling (nipple), localized edema
Neuromuscular & skeletal: Arthritis
Ophthalmic: Allergic conjunctivitis, blepharitis, diplopia, eye pain, optic neuritis, papilledema, photophobia, retinopathy (chorioretinopathy), swelling of eye
Otic: Auditory impairment, otalgia, tinnitus
Renal: Renal failure syndrome
Respiratory: Pulmonary hypertension, wheezing
Miscellaneous: Cyst (dermal), troponin increased in blood specimen
<1%, postmarketing, and/or case reports: Acute myocardial infarction, arteriosclerosis, ascorbic acid deficiency (Oak 2016), blurred vision, bronchitis, candidiasis, cardiac failure, cerebral infarction, chills, conjunctival hemorrhage, coronary artery disease, cyanosis, decreased visual acuity, dehydration, dental discomfort (sensitivity), dependent edema, disturbance in attention, dysuria, ecchymoses, erectile dysfunction, esophageal pain, exfoliative dermatitis, eye irritation, facial edema, fixed drug eruption, flu-like symptoms, gastritis, gastroesophageal reflux disease, gout, gynecomastia, heart murmur, hematoma, hepatotoxicity, hyperemia (scleral, conjunctival, ocular), hyperesthesia, hypertensive crisis, hyperthyroidism, hypothyroidism, increased appetite, increased blood urea nitrogen, increased lactate dehydrogenase, increased serum creatinine, intermittent claudication, interstitial pulmonary disease, intracranial hemorrhage, ischemic stroke, jaundice, joint swelling, local alterations in temperature sensations, loss of consciousness, mastalgia, melena, migraine, nocturia, oral mucosa ulcer, periorbital edema, pharyngolaryngeal pain, photopsia, pleurisy, pleuritic chest pain, pneumonia, pulmonary edema, skin pain, stiffness, stomatitis, syncope, throat irritation, thrombotic microangiopathy, toxic hepatitis, transient ischemic attacks, tremor, tumor lysis syndrome, urinary tract infection, urinary urgency, visual impairment, xerostomia
Warnings/Precautions
Concerns related to adverse effects:
- Bone marrow suppression: Reversible myelosuppression, including grades 3 and 4 thrombocytopenia, neutropenia, and anemia may occur; may require dose reductions and/or treatment delay. Monitor blood counts every 2 weeks for the first 2 months, then monthly.
- Cardiovascular: Cardiovascular events such as ischemic heart disease-related events, arterial vascular occlusive events, peripheral arterial occlusive disease, and ischemic cerebrovascular accident have been reported. Use caution in patients with preexisting risk factors, and monitor for new or worsening symptoms suggestive of cardiovascular events.
- Electrolyte imbalance: Electrolyte abnormalities may occur during treatment, including hypophosphatemia, hyper-/hypokalemia, hypocalcemia, and hyponatremia. Correct electrolyte abnormalities prior to treatment initiation; monitor periodically.
- Fluid retention: Fluid retention, including pleural and pericardial effusions, ascites, and pulmonary edema, were reported; may be severe. Monitor closely for signs/symptoms of fluid retention (eg, rapid weight gain or swelling) and for symptoms of respiratory or cardiac distress (eg, shortness of breath). Evaluate promptly and manage as appropriate.
- Hemorrhage: Serious hemorrhagic events (including fatal events) have occurred in chronic myelogenous leukemia (CML) patients treated with nilotinib. In a clinical study comparing nilotinib and imatinib in the treatment of newly diagnosed Philadelphia chromosome-positive (Ph+) chronic phase CML, hemorrhagic events (eg, GI hemorrhage, including grade 3 or 4 events) occurred more frequently in the nilotinib arm. Monitor for signs/symptoms of bleeding and manage as clinically appropriate.
- Hepatotoxicity: Nilotinib may cause hepatotoxicity, including elevations in bilirubin, transaminases, and alkaline phosphatase; grade 3 or 4 bilirubin, AST, and ALT elevations were observed more frequently in pediatric versus adult patients. Monitor liver function monthly or as clinically indicated.
- QT prolongation/sudden death: [US Boxed Warning]: May prolong the QT interval; sudden deaths have been reported. Use in patients with hypokalemia, hypomagnesemia, or long QT syndrome is contraindicated. Correct hypomagnesemia and hypokalemia prior to initiating therapy; monitor electrolytes periodically. Monitor ECG and QTc (baseline, at 7 days, with dose change, and periodically). Avoid the use of QT-prolonging agents and strong CYP3A4 inhibitors; also avoid concurrent use with antiarrhythmics; may increase the risk of potentially fatal arrhythmias. The sudden deaths reported appear to be related to dose-dependent ventricular repolarization abnormalities. Prolonged QT interval may result in torsade de pointes, which may cause syncope, seizure, and/or death. Patients with uncontrolled or significant cardiovascular disease were excluded from studies.
- Tumor lysis syndrome: Tumor lysis syndrome (TLS) has been reported in patients with resistant or intolerant CML; the majority of cases had malignant disease progression, high WBC counts, and/or dehydration. Maintain adequate hydration and treat high uric acid levels prior to nilotinib initiation.
Disease-related concerns:
- Gastrectomy: Consider alternative therapy or a dosage increase (with more frequent monitoring) in patients with total gastrectomy (nilotinib exposure is reduced).
- Hepatic impairment: Dosage reduction is recommended in patients with hepatic impairment, along with close monitoring of QT interval. Nilotinib metabolism is primarily hepatic; exposure is increased in patients with hepatic impairment.
- Pancreatitis: Use with caution in patients with a history of pancreatitis, may cause dose-limiting elevations of serum lipase and amylase; monitor. In patients with abdominal symptoms in conjunction with lipase increases, withhold treatment and consider diagnostics to exclude pancreatitis. Monitor serum lipase levels monthly or as clinically necessary.
Concurrent drug therapy issues:
- Drug-drug/drug-food interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information. [US Boxed Warning]: Avoid concurrent use with medications known to prolong the QT interval and with strong CYP3A4 inhibitors.
Special populations:
- Pediatrics: Growth retardation has been reported in pediatric patients with Ph+ CML in chronic phase. Monitor bone growth/development in pediatric patients.
- Polymorphisms: UGT1A1 polymorphisms may be a risk factor for increased toxicity (eg, hyperbilirubinemia) (Shibata 2014).
Dosage form specific issues:
- Lactose: Capsules contain lactose; do not use with galactose intolerance, severe lactase deficiency, or glucose-galactose malabsorption syndromes.
Other warnings/precautions:
- Appropriate administration: [US Boxed Warning]: Administer on an empty stomach, at least 1 hour before and 2 hours after food. Food increased the bioavailability/serum levels which may then prolong QTc. Nilotinib solubility is decreased at higher pH; concurrent use with proton pump inhibitors is not recommended. If necessary, H2-receptor blockers may be administered ~10 hours before and 2 hours after a nilotinib dose. Antacids (eg, aluminum hydroxide, magnesium hydroxide, simethicone) may be administered ~2 hours before or 2 hours after nilotinib.
- BCR-ABL transcript monitoring: Monitor BCR-ABL transcript levels in patients eligible for treatment discontinuation using an authorized test validated to measure molecular response levels with a sensitivity of at least MR4.5 (BCR-ABL/ABL ≤0.0032% IS). Information on authorized tests for detection and quantitation of BCR-ABL transcripts is available at http://www.fda.gov/CompanionDiagnostics.
Monitoring Parameters
Philadelphia chromosome status (prior to treatment); CBC with differential (every 2 weeks for first 2 months, then monthly); electrolytes (including potassium, calcium, and magnesium; baseline and periodic); lipid profile and glucose (baseline and periodically during the first year, then at least yearly), hepatic function (ALT/AST, bilirubin, alkaline phosphatase; baseline and monthly or as clinically indicated); serum lipase/amylase (baseline and monthly or as clinically indicated), uric acid (baseline); bone marrow assessments; pregnancy test (in females of reproductive potential; prior to initiating therapy); ECG and QTc (baseline, 7 days after treatment initiation or dosage adjustments, and periodically thereafter); signs/symptoms of cardiovascular events, hemorrhage, or fluid retention; monitor growth and development in pediatric patients.
In patients who discontinue nilotinib after a sustained molecular response (MR4.5), monitor BCR-ABL transcript levels and CBC with differential monthly for 1 year, then every 6 weeks for the second year, and every 12 weeks thereafter. Upon the loss of MR4.0 (corresponding to BCR-ABL/ABL ≤0.01% IS) during the treatment-free phase, monitor BCR-ABL transcript levels every 2 weeks until the BCR-ABL levels remain lower than major molecular response (MMR, corresponding to MR3.0 or BCR-ABL/ABL ≤0.1% IS) for 4 consecutive measurements; patients can then proceed to the original monitoring schedule. In patients who have reinitiated nilotinib after loss of molecular response, monitor CBC and BCR-ABL transcript levels every 4 weeks until a major molecular response (MR4.0) is re-established, and then every 12 weeks thereafter.
Thyroid function testing (Hamnvik 2011):
Preexisting levothyroxine therapy: Obtain baseline thyroid-stimulating hormone (TSH) levels, then monitor every 4 weeks until levels and levothyroxine dose are stable, then monitor every 2 months
Without preexisting thyroid hormone replacement: TSH at baseline, then monthly for 4 months, then every 2 to 3 months
Monitor adherence.
Pregnancy
Pregnancy Considerations
Based on data from animal reproduction studies and the mechanism of action, nilotinib may cause fetal harm if administered during pregnancy. Verify pregnancy status in females of reproductive potential prior to initiating therapy. Women of reproductive potential should be advised to use effective contraception during treatment and for at least 14 days after the last dose.
Patient Education
What is this drug used for?
- It is used to treat leukemia.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Nausea
- Vomiting
- Abdominal pain
- Lack of appetite
- Constipation
- Diarrhea
- Joint pain
- Muscle pain
- Back pain
- Hair loss
- Trouble sleeping
- Common cold symptoms
- Stuffy nose
- Sore throat
- Itching
- Night sweats
- Dry skin
- Muscle spasms
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Infection
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Pancreatitis like severe abdominal pain, severe back pain, severe nausea, or vomiting.
- High blood sugar like confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit.
- Bleeding like vomiting blood or vomit that looks like coffee grounds; coughing up blood; blood in the urine; black, red, or tarry stools; bleeding from the gums; abnormal vaginal bleeding; bruises without a reason or that get bigger; or any severe or persistent bleeding.
- Electrolyte problems like mood changes, confusion, muscle pain or weakness, abnormal heartbeat, seizures, lack of appetite, or severe nausea or vomiting.
- Severe cerebrovascular disease like change in strength on one side is greater than the other, difficulty speaking or thinking, change in balance, or vision changes.
- Chest pain
- Fast heartbeat
- Passing out
- Abnormal heartbeat
- Dizziness
- Shortness of breath
- Excessive weight gain
- Swelling of arms or legs
- Vision changes
- Severe headache
- Severe loss of strength and energy
- Leg pain
- Cold sensation of the legs
- Skin discoloration of the leg
- Tumor lysis syndrome like fast heartbeat or abnormal heartbeat; any passing out; unable to pass urine; muscle weakness or cramps; nausea, vomiting, diarrhea or lack of appetite; or feeling sluggish.
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.