What is Oxbryta?
Oxbryta is a prescription medicine used for the treatment of sickle cell disease in adults and children 4 years of age and older.
It is not known if Oxbryta is safe and effective in children with sickle cell disease below 4 years of age.
Who should not take Oxbryta?
Do not take Oxbryta if you or your child have had an allergic reaction to voxelotor or any of the ingredients in Oxbryta. See the end of this leaflet for a list of the ingredients in Oxbryta.
What should I tell my healthcare provider before taking Oxbryta?
Before taking Oxbryta, tell your healthcare provider about all of your medical conditions, including if you or your child:
- have liver problems
- are pregnant or plan to become pregnant. It is not known if Oxbryta can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Oxbryta can pass into your breastmilk and if it can harm your baby. Do not breastfeed during treatment with Oxbryta and for at least 2 weeks after the last dose.
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how Oxbryta works. Oxbryta may also affect how other medicines work and may affect the results of certain blood tests. Keep a list of all your medicines and show it to your healthcare provider.
How should I take Oxbryta?
- Take Oxbryta exactly as your healthcare provider tells you.
- Do not change your dose or stop taking Oxbryta unless your healthcare provider tells you to. Your healthcare provider may change your dose if needed.
- Take your prescribed dose of Oxbryta 1 time each day.
- Take Oxbryta with or without food.
- Oxbryta comes in two different dosage forms, Oxbryta tablets and Oxbryta tablets for oral suspension. Your healthcare provider will decide which dosage form you take based on your age, weight, and ability to swallow tablets.
- If you take Oxbryta tablets: Swallow each Oxbryta tablet whole. Do not cut, crush, or chew the tablets.
- If you take Oxbryta tablets for oral suspension: See the detailed Instructions for use on how to prepare and take your dose. You must mix the Oxbryta tablets for oral suspension in room temperature clear drink, such as drinking water or clear soda, right before taking it. Do not swallow whole, cut, crush, or chew the tablets for oral suspension.
- Check to make sure you receive the correct dosage form of Oxbryta each time your prescription is filled to avoid taking the wrong medicine.
- Your healthcare provider may also prescribe a medicine called hydroxyurea during treatment with Oxbryta.
- If you or your child miss a dose of Oxbryta or if the entire dose is not taken, skip that dose and return to your normal dosing schedule the next day.
What should I avoid while taking Oxbryta?
Do not take St. John's wort during treatment with Oxbryta.
What are the possible side effects of Oxbryta?
Oxbryta can cause serious side effects, including:
- Serious allergic reactions. Tell your healthcare provider or get emergency medical help right away if you get:
- rash
- hives
- shortness of breath (difficult breathing)
- swelling of the face
The most common side effects of Oxbryta include:
- headache
- diarrhea
- stomach-area (abdominal) pain
- nausea
- rash or hives
- fever
The most common side effects of Oxbryta in children ages 4 to less than 12 years of age include:
- fever
- vomiting
- rash
- stomach-area (abdominal) pain
- diarrhea
- headache
These are not all the possible side effects of Oxbryta.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Global Blood Therapeutics, Inc. at 1-833-428-4968 (1-833-GBT-4YOU).
Oxbryta Images
General information about the safe and effective use of Oxbryta
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Oxbryta for a condition for which it was not prescribed. Do not give Oxbryta to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Oxbryta that is written for health professionals.
How should I store Oxbryta?
- Store Oxbryta between 68°F to 86°F (20°C to 30°C).
- The Oxbryta bottle comes with a child-resistant closure.
- The Oxbryta bottle may contain a desiccant canister to help keep your medicine dry (protect it from moisture) and a polyester coil. Do not eat the desiccant or polyester coil.
Keep Oxbryta and all medicines out of the reach of children.
What are the ingredients in Oxbryta?
Active Ingredient: voxelotor
Inactive Ingredients:
Tablets: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The film coating contains: polyethylene glycol 3350, polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
Tablets for oral suspension: artificial grape flavor, colloidal silicon dioxide, croscarmellose sodium, iron oxide pigment, magnesium stearate, microcrystalline cellulose, and sucralose.
For more information, call 1-833-428-4968 (1-833-GBT-4YOU) or go to www.Oxbryta.com.
Instructions for use for Oxbryta
Oxbryta [ox brye ta]
(voxelotor)
tablets for oral suspension
300 mg
This Instructions for Use contains information on how to take Oxbryta tablets for oral suspension.
Read this Instructions for Use before you or your child start taking Oxbryta tablets for oral suspension for the first time and each time you or your child get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your or your child's medical condition or treatment.
Talk to your healthcare provider or pharmacist if you have questions about how to take or give the prescribed dose of Oxbryta tablets for oral suspension.
Important information you need to know before taking oxbryta tablets for oral suspension:
- Take Oxbryta tablets for oral suspension exactly as your healthcare provider tells you.
- Your healthcare provider will tell you how many Oxbryta tablets for oral suspension you will need for your or your child's dose.
- Oxbryta comes in two different dosage forms. Each time you receive your or your child's prescription, check the bottle to make sure that you received the prescribed dosage form of Oxbryta tablets for oral suspension. Oxbryta tablets for oral suspension are light yellow to yellow, round shaped, and marked with "300 D" on one side. Contact your pharmacist or healthcare provider if you did not receive the correct dosage form.
- Do not swallow whole, cut, crush, or chew Oxbryta tablets for oral suspension.
- You must first mix Oxbryta tablets for oral suspension in room temperature clear drink, such as drinking water or clear soda, before swallowing.
- If you or your child miss a dose of Oxbryta tablets for oral suspension or if the entire dose is not taken, skip that dose and return to the normal dosing schedule the next day.
- For more information about Oxbryta tablets for oral suspension, see the information above.
Gather supplies
You will need the following items to prepare the dose of Oxbryta tablets for oral suspension (not included with Oxbryta tablets for oral suspension):
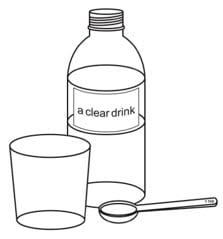
- a teaspoon
- a small cup
- room temperature clear drink, such as drinking water or clear soda.
You will also need:
- the prescribed number of Oxbryta tablets for oral suspension needed for your dose.
Preparing a dose of Oxbryta tablets for oral suspension
Step 1. Wash and dry your hands well before preparing the dose.
Step 2. Pour room temperature clear drink into the cup. The table below shows the amount of clear drink needed for your prescribed dose. You may add more clear drink if needed to mix the tablets.
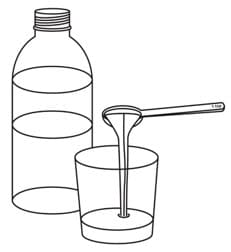
| Number of Oxbryta Tablets for Oral Suspension | Amount of Clear Drink |
| 1 | 1 teaspoon (5 mL) |
| 2 | 2 teaspoons (10 mL) |
| 3 | 3 teaspoons (15 mL) |
| 4 | 4 teaspoons (20 mL) |
| 5 | 5 teaspoons (25 mL) |
| 7 | 7 teaspoons (35 mL) |
| 8 | 8 teaspoons (40 mL) |
Step 3. Add the prescribed number of Oxbryta tablets for oral suspension into the cup.
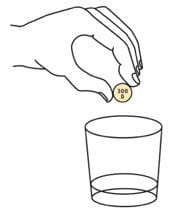
Step 4. Swirl the cup until the tablet(s) break apart (disperse) in the drink. Be careful not to spill the mixture.
- The tablet(s) will not completely dissolve. You will still see small tablet clumps in the mixture.
- If you spill any medicine, clean up the spill. Throw away the rest of the prepared medicine and make a new dose.
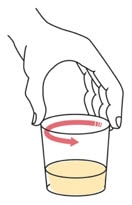
Step 5. Wait for 1 to 5 minutes.

Giving the dose
Step 6. Swirl the cup again. Take or give all of the prepared medicine right away.
- If giving to a child, make sure that your child is upright when drinking the medicine mixture.
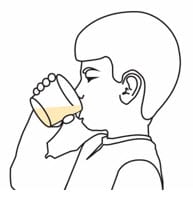
Step 7. Add 1 or 2 teaspoons of room temperature clear drink to the cup to make sure the full dose is taken. Swirl the cup until the remaining medicine is mixed and take or give it right away.
- Repeat this step until no more medicine is left in the cup.
- After all the medicine is taken, you or your child may drink more water or any type of drink.
Step 8. Wash the teaspoon and cup with warm soap and water.
Storing Oxbryta
- Store Oxbryta between 68°F to 86°F (20°C to 30°C).
- The Oxbryta bottle comes with a child-resistant closure.
- The Oxbryta tablets for oral suspension bottle contains a polyester coil. Do not eat the polyester coil.
Keep Oxbryta and all medicines out of the reach of children.
Disposing of Oxbryta
When all the tablets in the bottle have been taken, throw away the bottle. Safely dispose of (throw away) Oxbryta that is out of date or no longer needed using your local household waste guidelines.
Instructions for use approved 12/2021.