Boxed Warning
Lactic acidosis and hepatomegaly with steatosis:
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including stavudine and other antiretrovirals. Fatal lactic acidosis has been reported in pregnant individuals who received the combination of stavudine and didanosine with other antiretroviral agents. Coadministration of stavudine and didanosine is contraindicated because of increased risk of serious and/or life-threatening events. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Pancreatitis:
Fatal and nonfatal pancreatitis has occurred during therapy when stavudine was part of a combination regimen that included didanosine in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Capsule, Oral:
Zerit: 15 mg [DSC], 20 mg [DSC], 30 mg [DSC], 40 mg [DSC]
Generic: 15 mg, 20 mg, 30 mg, 40 mg
Solution Reconstituted, Oral:
Zerit: 1 mg/mL (200 mL [DSC]) [dye free; fruit flavor]
Pharmacology
Mechanism of Action
Stavudine is a thymidine analog which interferes with HIV viral DNA dependent DNA polymerase resulting in inhibition of viral replication; nucleoside reverse transcriptase inhibitor
Pharmacokinetics/Pharmacodynamics
Absorption
Rapid
Distribution
Penetrates into the CSF achieving 16% to 97% (mean: 59%) of concomitant plasma concentrations; distributes into extravascular spaces and equally between RBCs and plasma
Vd: Children: 0.73 ± 0.32 L/kg; Adults: 46 ± 21 L
Metabolism
Converted intracellularly to active triphosphate form; metabolism of stavudine plays minimal role in its clearance; minor metabolites include oxidized stavudine and its glucuronide conjugate, glucuronide conjugate of stavudine, N-acetylcysteine conjugate of the ribose after glycosidic cleavage
Excretion
Urine 95% (74% as unchanged drug); feces 3% (62% as unchanged drug)
Time to Peak
Serum: 1 hour
Half-Life Elimination
Note: Half-life is prolonged with renal dysfunction
Newborns (at birth): 5.3 ± 2 hours
Neonates 14 to 28 days old: 1.6 ± 0.3 hours
Children 5 weeks to 15 years: 0.9 ± 0.3 hours
Adults: 1.6 ± 0.2 hours
Intracellular: Adults: 3.5 to 7 hours
Protein Binding
Negligible
Use in Specific Populations
Special Populations: Renal Function Impairment
Oral Cl decreases and terminal elimination half-life increases in patients with renal insufficiency. Adjust dosage in patients with reduced CrCl and patients receiving maintenance hemodialysis.
Use: Labeled Indications
HIV-1: Treatment of HIV-1 infection in combination with other antiretroviral agents
Contraindications
Hypersensitivity to stavudine or any component of the formulation; coadministration with didanosine
Dosage and Administration
Dosing: Adult
HIV-1 infection, treatment: Oral:
<60 kg: 30 mg every 12 hours
≥60 kg: 40 mg every 12 hours
Note: According to the Department and Health and Human Services (HHS) HIV treatment guidelines, the World Health Organization recommends 30 mg every 12 hours regardless of body weight (HHS [adult] 2016).
Dosing: Geriatric
Older patients should be closely monitored for signs and symptoms of peripheral neuropathy. Dosage should be carefully adjusted to renal function.
Dosing: Pediatric
Note: Gene mutation and antiretroviral (ARV) resistance patterns should be evaluated (refer to www.iasusa.org for more information) when necessary.
HIV-1 infection, treatment: Note: Although FDA approved, stavudine is no longer recommended for use in pediatric patients due to higher rates of adverse effects than other nucleoside reverse transcriptase inhibitors (HHS [adult, pediatric] 2018). If used, it should be in combination with other ARV agents.
Infants and Children <30 kg: Oral: 1 mg/kg/dose every 12 hours; maximum dose: 30 mg/dose.
Children and Adolescents weighing 30 to <60 kg: Oral: 30 mg every 12 hours.
Adolescents weighing ≥60 kg: AIDSInfo and WHO recommendation: Oral: 30 mg every 12 hours (HHS [pediatric] 2018). Note: The manufacturer's labeling (40 mg) is not recommended due to a greater incidence of adverse effects (HHS [pediatric] 2018).
Reconstitution
Reconstitute powder for oral suspension with 202 mL of purified water as specified on the bottle. Shake vigorously until suspended. Final suspension will be 1 mg/mL (200 mL).
Administration
May be administered without regard to meals. Capsule may be opened and dispersed in a small amount of water; administer immediately (HHS [pediatric] 2016). Oral solution should be shaken vigorously prior to use.
Dietary Considerations
May be taken without regard to meals. Some products may contain sucrose.
Storage
Capsules and powder for reconstitution may be stored at controlled room temperature of 25°C (77°F). Reconstituted oral solution should be stored in refrigerator at 2°C to 8°C (36°F to 46°F) and is stable for 30 days.
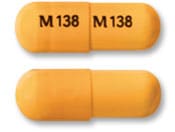
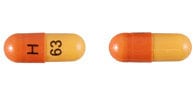
Stavudine Images
Drug Interactions
Cladribine: Agents that Undergo Intracellular Phosphorylation may diminish the therapeutic effect of Cladribine. Avoid combination
Didanosine: Stavudine may enhance the adverse/toxic effect of Didanosine. The risk of lactic acidosis (possibly fatal), hepatomegaly, and pancreatitis may be increased with this combination. Avoid combination
DOXOrubicin (Conventional): May diminish the therapeutic effect of Stavudine. Monitor therapy
DOXOrubicin (Liposomal): May diminish the therapeutic effect of Stavudine. Monitor therapy
Hydroxyurea: May enhance the adverse/toxic effect of Stavudine. An increased risk of pancreatitis, hepatotoxicity and/or neuropathy may exist. Stavudine may enhance the adverse/toxic effect of Hydroxyurea. An increased risk of pancreatitis, hepatotoxicity and/or neuropathy may exist. Avoid combination
Levomethadone: May decrease the serum concentration of Stavudine. Monitor therapy
Methadone: May decrease the serum concentration of Stavudine. Monitor therapy
Orlistat: May decrease the serum concentration of Antiretroviral Agents. Monitor therapy
Zidovudine: May diminish the therapeutic effect of Stavudine. Avoid combination
Adverse Reactions
Adverse reactions reported below represent experience with combination therapy with other nucleoside analogues and protease inhibitors.
>10%:
Central nervous system: Headache (25% to 46%), peripheral neuropathy (8% to 21%)
Dermatologic: Skin rash (18% to 30%)
Endocrine & metabolic: Increased amylase (21% to 31%; grades 3/4: 4% to 8%), increased gamma-glutamyl transferase (15% to 28%; grades 3/4: 2% to 5%)
Gastrointestinal: Nausea (43% to 53%), diarrhea (34% to 45%), vomiting (18% to 30%), increased serum lipase (27%; grades 3/4: 5% to 6%)
Hepatic: Hyperbilirubinemia (65% to 68%; grades 3/4: 7% to 16%), increased serum AST (42% to 53%; grades 3/4: 5% to 7%), increased serum ALT (40% to 50%; grades 3/4: 6% to 8%)
<1%, postmarketing, and/or case reports: Abdominal pain, anemia, anorexia, chills, diabetes mellitus, fever, hepatic failure, hepatitis, hepatomegaly with steatosis (some fatal), hyperglycemia, hyperlipidemia, hypersensitivity reaction, immune reconstitution syndrome, insomnia, insulin resistance, lactic acidosis (some fatal), leukopenia, lipoatrophy, lipotrophy, macrocytosis, myalgia, neutropenia, pancreatitis (some fatal), redistribution of body fat, severe weakness (severe neuromuscular weakness resembling Guillain-Barré), thrombocytopenia
Warnings/Precautions
Concerns related to adverse effects:
- Immune reconstitution syndrome: Patients may develop immune reconstitution syndrome resulting in the occurrence of an inflammatory response to an indolent or residual opportunistic infection during initial HIV treatment or activation of autoimmune disorders (eg, Graves’ disease, polymyositis, Guillain-Barré syndrome) later in therapy; further evaluation and treatment may be required.
- Lactic acidosis/hepatomegaly: [US Boxed Warning]: Lactic acidosis and severe hepatomegaly with steatosis have been reported with nucleoside analogues, including fatal cases; coadministration of stavudine and didanosine is contraindicated. Use with caution in patients with risk factors for liver disease (although acidosis has occurred in patients without known risk factors, risk may be increased with female gender, obesity, pregnancy, or prolonged exposure). Suspend treatment in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or hepatotoxicity (transaminase elevation may/may not accompany hepatomegaly and steatosis).
- Lipoatrophy: May cause loss of subcutaneous fat, especially in the face, limbs, and buttocks. Lipoatrophy incidence and severity are related to cumulative exposure and may be only partially reversible. Monitor patients for signs of lipoatrophy and consider switching to a nonstavudine-containing regimen if lipoatrophy occurs.
- Motor weakness: Severe motor weakness (resembling Guillain-Barré syndrome) has been reported (including fatal cases, usually in association with lactic acidosis); manufacturer recommends discontinuation if motor weakness develops (with or without lactic acidosis).
- Pancreatitis: [US Boxed Warning]: Pancreatitis (including some fatal cases) has occurred during combination therapy with didanosine. Coadministration of stavudine and didanosine is contraindicated. Suspend stavudine and any agents toxic to the pancreas in patients with suspected pancreatitis. If pancreatitis diagnosis is confirmed, use extreme caution if reinitiating stavudine; monitor closely and do not use didanosine in regimen.
- Peripheral neuropathy: May be treatment-limiting, especially with higher doses; use with caution in patients with pre-existing peripheral neuropathy, advanced HIV, and/or in combination with other medications known to cause neuropathy. Consider discontinuation of therapy if peripheral neuropathy develops; effect may be reversible if therapy discontinued immediately. Symptoms may worsen initially when therapy is discontinued.
Disease-related concerns:
- Bone marrow suppression: Use with caution in patients with pre-existing bone marrow suppression.
- Hepatic impairment: Use with caution in patients with hepatic impairment; discontinue or interrupt therapy if worsening liver function occurs.
- Renal impairment: Use with caution in patients with renal impairment; dosage adjustment recommended.
Concurrent drug therapy issues:
- Combination with didanosine or hydroxyurea: May increase risk of hepatotoxicity, pancreatitis, or severe peripheral neuropathy; lactic acidosis may also occur with concomitant didanosine administration. Use with hydroxyurea should be avoided; coadministration with didanosine is contraindicated.
- Interferon alfa: Use with caution in combination with interferon alfa with or without ribavirin in HIV/HBV coinfected patients; monitor closely for hepatic decompensation, anemia, or neutropenia; dose reduction or discontinuation of interferon and/or ribavirin may be required if toxicity evident.
- Zidovudine: Should not use zidovudine in combination with stavudine.
Monitoring Parameters
Monitor liver function tests and renal function tests; signs and symptoms of peripheral neuropathy; monitor viral load and CD4 count
Pregnancy
Pregnancy Considerations
Stavudine crosses the human placenta.
[US Boxed Warning]: Fatal lactic acidosis has been reported in pregnant individuals using didanosine and stavudine in combination with other antiretroviral agents; coadministration of stavudine and didanosine is contraindicated.
Outcome information specific to stavudine use in pregnancy is no longer being reviewed and updated in the Health and Humans Services (HHS) perinatal guidelines. Maternal antiretroviral therapy (ART) may be associated with adverse pregnancy outcomes, including preterm delivery, stillbirth, low birth weight, and small-for-gestational-age infants. Actual risks may be influenced by maternal factors such as disease severity, gestational age at initiation of therapy, and specific ART regimen; therefore, close fetal monitoring is recommended. Because there is clear benefit to appropriate treatment, maternal ART should not be withheld due to concerns for adverse neonatal outcomes. Long-term follow-up is recommended for all infants exposed to antiretroviral medications; children without HIV but who were exposed to ART in utero and develop significant organ system abnormalities of unknown etiology (particularly of the CNS or heart) should be evaluated for potential mitochondrial dysfunction.
Based on the HHS perinatal HIV guidelines, stavudine is not one of the recommended antiretroviral agents for use during pregnancy or females living with HIV who are trying to conceive.
In general, ART is recommended for all pregnant females living with HIV to keep the viral load below the limit of detection and reduce the risk of perinatal transmission. Therapy should be individualized following a discussion of the potential risks and benefits of treatment during pregnancy. Monitoring of pregnant females is more frequent than in nonpregnant adults. ART should be continued postpartum for all females living with HIV and can be modified after delivery.
Health care providers are encouraged to enroll pregnant females exposed to antiretroviral medications as early in pregnancy as possible in the Antiretroviral Pregnancy Registry (1-800-258-4263 or http://www.APRegistry.com). Health care providers caring for pregnant females living with HIV and their infants may contact the National Perinatal HIV Hotline (888-448-8765) for clinical consultation (HHS [perinatal] 2019).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience headache, nausea, vomiting, or diarrhea. Have patient report immediately to prescriber signs of pancreatitis (severe abdominal pain, severe back pain, severe nausea, or vomiting), signs of lactic acidosis (fast breathing, fast heartbeat, abnormal heartbeat, vomiting, fatigue, shortness of breath, severe loss of strength and energy, severe dizziness, feeling cold, or muscle pain or cramps), signs of liver problems (dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin), signs of infection, muscle weakness, burning or numbness feeling, or change in body fat (HCAHPS)
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.