What is Stimufend?
Stimufend is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is a substance produced by the body. It stimulates the growth of neutrophils, a type of white blood cell important in the body's fight against infection.
Who should not use Stimufend?
Do not take Stimufend if you have had a serious allergic reaction to pegfilgrastim products or filgrastim products.
What should I tell my healthcare provider before using Stimufend?
Before you receive Stimufend, tell your healthcare provider about all of your medical conditions, including if you:
- have a sickle cell disorder.
- have kidney problems.
- are allergic to latex. The needle cap on the prefilled syringe contains dry natural rubber (derived from latex). You should not give Stimufend using the prefilled syringe if you have latex allergies.
- are pregnant or plan to become pregnant. It is not known if Stimufend will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Stimufend passes into your breast milk.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Stimufend?
- Stimufend is given as an injection under your skin (subcutaneous injection) by a healthcare provider. If your healthcare provider decides that the subcutaneous injections can be given at home by you or your caregiver, follow the detailed “Instructions for use” that comes with your Stimufend for information on how to prepare and inject a dose of Stimufend.
- You and your caregiver will be shown how to prepare and inject Stimufend before you use it.
- You should not inject a dose of Stimufend to children weighing less than 45 kg from a Stimufend prefilled syringe. A dose less than 0.6 mL (6 mg) cannot be accurately measured using the Stimufend prefilled syringe.
- If you are receiving Stimufend because you are also receiving chemotherapy, the last dose of Stimufend should be injected at least 14 days before and 24 hours after your dose of chemotherapy.
- If you miss a dose of Stimufend, talk to your healthcare provider about when you should give your next dose.
What are the possible side effects of Stimufend?
Stimufend may cause serious side effects, including:
- Spleen rupture. Your spleen may become enlarged and can rupture. A ruptured spleen can cause death. Call your healthcare provider right away if you have pain in the left upper stomach area or your left shoulder.
- A serious lung problem called Acute Respiratory Distress Syndrome (ARDS). Call your healthcare provider or get emergency help right away if you have shortness of breath with or without a fever, trouble breathing, or a fast rate of breathing.
- Serious allergic reactions. Stimufend can cause serious allergic reactions. These reactions can cause a rash over your whole body, shortness of breath, wheezing, dizziness, swelling around your mouth or eyes, fast heart rate, and sweating. If you have any of these symptoms, stop using Stimufend and call your healthcare provider or get emergency medical help right away.
- Sickle cell crises. You may have a serious sickle cell crisis, which could lead to death, if you have a sickle cell disorder and receive Stimufend. Call your healthcare provider right away if you have symptoms of sickle cell crisis such as pain or difficulty breathing.
- Kidney injury (glomerulonephritis). Stimufend can cause kidney injury. Call your healthcare provider right away if you develop any of the following symptoms:
- swelling of your face or ankles
- blood in your urine or dark colored urine
- you urinate less than usual
- Increased white blood cell count (leukocytosis). Your healthcare provider will check your blood during treatment with Stimufend.
- Decreased platelet count (thrombocytopenia). Your healthcare provider will check your blood during treatment with Stimufend. Tell your healthcare provider if you have unusual bleeding or bruising during treatment with Stimufend. This could be a sign of decreased platelet counts, which may reduce the ability of your blood to clot.
- Capillary Leak Syndrome. Stimufend can cause fluid to leak from blood vessels into your body's tissues. This condition is called “Capillary Leak Syndrome” (CLS). CLS can quickly cause you to have symptoms that may become life-threatening. Get emergency medical help right away if you develop any of the following symptoms:
- swelling or puffiness and are urinating less than usual
- trouble breathing
- swelling of your stomach area (abdomen) and feeling of fullness
- dizziness or feeling faint
- a general feeling of tiredness
- Myelodysplastic syndrome and acute myeloid leukemia. If you have breast cancer or lung cancer, when Stimufend is used with chemotherapy and radiation therapy, or with radiation therapy alone, you may have an increased risk of developing a precancerous blood condition called myelodysplastic syndrome (MDS) or a blood cancer called acute myeloid leukemia (AML). Symptoms of MDS and AML may include tiredness, fever, and easy bruising or bleeding. Call your healthcare provider if you develop these symptoms during treatment with Stimufend.
- Inflammation of the aorta (aortitis). Inflammation of the aorta (the large blood vessel which transports blood from the heart to the body) has been reported in patients who received pegfilgrastim products. Symptoms may include fever, abdominal pain, feeling tired, and back pain. Call your healthcare provider if you experience these symptoms.
The most common side effects of Stimufend are pain in the bones, arms, and legs.
These are not all the possible side effects of Stimufend. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Stimufend
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Stimufend for a condition for which it was not prescribed. Do not give Stimufend to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Stimufend that is written for health professionals.
How should I store Stimufend?
- Store Stimufend in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze. Throw away (dispose of) Stimufend that has been frozen.
- Keep the prefilled syringe in the original carton to protect from light or physical damage.
- Do not shake the prefilled syringe.
- Take Stimufend out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- Throw away (dispose of) any Stimufend that has been left at room temperature, 68°F to 77°F (20°C to 25°C), for more than 72 hours.
Keep the Stimufend prefilled syringe out of the reach of children.
What are the ingredients in Stimufend?
Active ingredient: pegfilgrastim-fpgk
Inactive ingredients: acetate, polysorbate 20, sodium and sorbitol in Water for Injection, USP.
For more information go to www.fresenius-kabi.com/us, or call 1-800-551-7176.
Instructions for use for Stimufend
Stimufend (STIM-yu-fend)
(pegfilgrastim-fpgk)
Injection, for subcutaneous use
Single-Dose Prefilled Syringe
Guide to parts
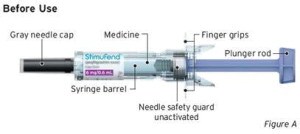
After Use (Clear needle guard locked in place)
Important: The needle is covered by a gray needle cap before use.
Important:
Read the Patient Information for important information you need to know about Stimufend before using these Instructions for Use.
Before you use a Stimufend prefilled syringe, read this important information.
Storing the prefilled syringe
- Store Stimufend in the refrigerator between 36°F and 46°F (2°C to 8°C).
- Do not freeze. Throw away (dispose of) Stimufend that has been frozen.
- Keep the prefilled syringe in the original carton to protect from light or physical damage.
- Take the prefilled syringe out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- Throw away (dispose of) any Stimufend that has been left at room temperature, 68°F to 77°F (20°C to 25°C), for more than 72 hours.
- Keep the Stimufend prefilled syringe out of the reach of children.
Using the prefilled syringe
- It is important that you do not try to give the injection unless you or your caregiver has received training from your healthcare provider.
- Make sure the name Stimufend appears on the carton and prefilled syringe label.
- Check the carton and prefilled syringe label to make sure the dose strength is 6 mg/0.6 mL.
- You should not inject a dose of Stimufend to children weighing less than 45 kg from a Stimufend prefilled syringe. A dose less than 0.6 mL (6 mg) cannot be accurately measured using the Stimufend prefilled syringe.
- Do not use a prefilled syringe after the expiration date on the label.
- Do not shake the prefilled syringe.
- Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
- Do not use the prefilled syringe if the carton is open or damaged.
- Do not use a prefilled syringe if it has been dropped on a hard surface. The prefilled syringe may be broken even if you cannot see the break. Use a new prefilled syringe.
- The gray needle cap on the prefilled syringe contains dry natural rubber (made from latex). Tell your healthcare provider if you are allergic to latex. You should not give Stimufend using the prefilled syringe if you have latex allergies.
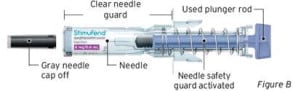
The prefilled syringe has a clear needle guard that automatically activates to cover the needle after the injection is given. Do not use a prefilled syringe if the clear needle guard has been activated. Use another prefilled syringe that has not been activated and is ready to use.
Call your healthcare provider if you have any questions.
Step 1: Prepare
1.1 Remove the prefilled syringe carton from the refrigerator. Remove the syringe tray from the carton.
On a clean, well-lit surface, place the syringe tray at room temperature for 30 minutes before you give an injection.
- Do not use the prefilled syringe if the carton is damaged.
- Do not try to warm the prefilled syringe by using a heat source such as hot water or microwave.
- Do not leave the prefilled syringe in direct sunlight.
- Do not shake the prefilled syringe.
Open the tray by peeling away the cover. Grab the clear needle guard to remove the prefilled syringe from the tray (see Figure C).
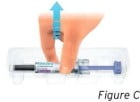
For safety reasons:
- Do not grab the plunger rod.
- Do not grab the gray needle cap.
1.2 Inspect the medicine and prefilled syringe.
Make sure the medicine in the prefilled syringe is clear and colorless.
- Do not use the prefilled syringe if:
- The needle safety guard is activated (see Figure D).
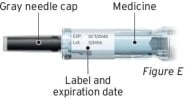
- The medicine is cloudy or discolored, or contains flakes or particles (see Figure E).
- Any part appears cracked or broken.
- The prefilled syringe has been dropped.
- The gray needle cap is missing or not securely attached (see Figure E).
- The expiration date printed on the label has passed (see Figure E).
In all cases use a new syringe and call your healthcare provider.
1.3 Gather all materials needed for the injection (see Figure F). Wash your hands thoroughly with soap and water.
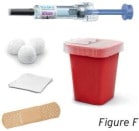
On a clean, well-lit work surface, place the:
- Prefilled syringe
- Alcohol wipe
- Cotton ball or gauze pad
- Adhesive bandage
- Sharps disposal container
Step 2: Get ready
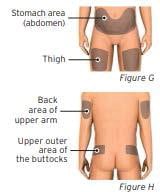
2.1 Prepare and clean the injection site(s). You can use:
- Stomach area (abdomen) except for a 2-inch area away from the navel (belly button) (see Figure G)
- Thigh (see Figure G)
- Back area of the upper arm (only if someone else is giving you the injection) (See Figure H)
- Upper outer area of the buttocks (only if someone else is giving you the injection) (see Figure H)
Clean the injection site with an alcohol wipe (see Figure I). Let the skin dry.

- Do not touch this area again before injecting.
- If you want to use the same injection site, make sure it is not the same spot on the injection site you used for a previous injection.
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
2.2 Hold the prefilled syringe by the clear needle guard. Carefully pull the gray needle cap straight off and away from the body (see Figure J).
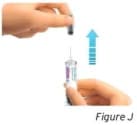
- Do not remove the gray needle cap from the prefilled syringe until you are ready to inject.
- Do not twist or bend the gray needle cap.
- Do not hold the prefilled syringe by the plunger rod.
- Do not put the gray needle cap back onto the prefilled syringe.
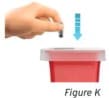
Important: Throw the gray needle cap into the sharps disposal container (see Figure K).
Step 3: Subcutaneous (under the skin) injection
3.1 Pinch the injection site to create a firm surface (see Figure L).
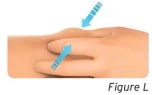
Important: Keep skin pinched while injecting.
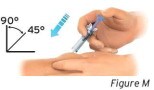
3.2 Hold the pinch. Insert the needle into the skin at 45 to 90 degrees (see Figure M).
3.3 Using slow and constant pressure, push the plunger rod until it reaches the bottom (see Figure N).
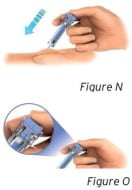
- The plunger rod must be pushed down fully to ensure the full dose has been injected (see Figure O).
- Do not remove the needle from the skin when the plunger reaches the end and proceed with next step.
Step 4: Finish
4.1 Slowly release your thumb upward. This will allow the needle to move up into the clear needle guard and cover the entire needle (see Figure P).
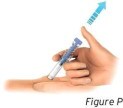
- Do not try to recap the needle as it could lead to needle stick injury.
Important: When you remove the syringe, if it looks like the medicine is still in the syringe barrel, this means you have not received a full dose. Call your healthcare provider right away.
4.2 Discard (throw away) your prefilled syringe.
- Put the used prefilled syringe in a FDA-cleared sharps disposal container right away after use (see Figure Q).

- Do not throw away (dispose of) the prefilled syringe in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not reuse the prefilled syringe.
- Do not recycle the prefilled syringe or sharps disposal container or throw them in the household trash.
Important: Always keep the sharps disposal container out of the reach of children.
4.3 Examine the injection site.
- If there is blood, press a cotton ball or gauze pad on the injection site. Do not rub the injection site. Apply an adhesive bandage if needed (see Figure R).

Instructions for use issued 09/2022




