Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
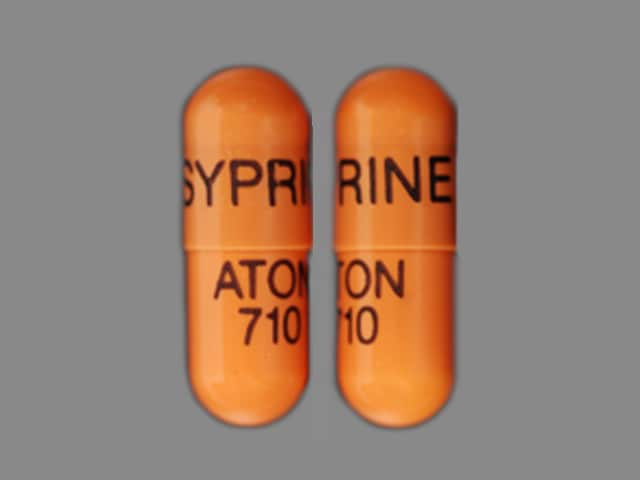
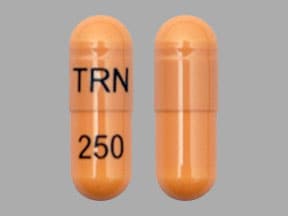
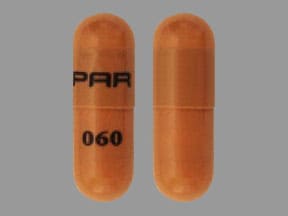
Capsule, Oral, as hydrochloride:
Clovique: 250 mg
Syprine: 250 mg
Generic: 250 mg
Pharmacology
Mechanism of Action
Trientine is an oral chelating agent structurally dissimilar from penicillamine and other available chelating agents; an effective oral chelator of copper used to induce adequate cupriuresis
Pharmacokinetics/Pharmacodynamics
Absorption
Poor (Roberts 2008)
Metabolism
To acetyltrien (chelating activity significantly less than parent) (Roberts 2008)
Excretion
Urine (1% as parent; 8% as metabolite) (Roberts 2008)
Use: Labeled Indications
Wilson disease: Treatment of patients with Wilson disease who are intolerant to penicillamine.
Limitations of use: Not recommended in cystinuria or rheumatoid arthritis; not indicated for biliary cirrhosis.
Contraindications
Hypersensitivity to trientine or any component of the formulation.
Dosage and Administration
Dosing: Adult
Wilson disease: Oral: Initial: 750 to 1,250 mg/day in divided doses 2 to 4 times daily; increase dose if clinical response not adequate or the concentration of free serum copper is persistently >20 mcg/dL; maximum dose: 2,000 mg/day. AASLD practice guidelines suggest typical doses of 750 to 1,500 mg/day in 2 to 3 divided doses with maintenance therapy of 750 to 1,000 mg/day (Roberts 2008). Optimal long-term maintenance dosage should be determined at 6- to 12-month intervals.
Dosing: Geriatric
Refer to adult dosing. Use with caution; initiate at lower end of the dosing range.
Dosing: Pediatric
Wilson disease: Children and Adolescents: Oral: Initial: 20 mg/kg/day (round dose to the nearest 250 mg) in 2 to 3 divided doses; maximum initial daily dose: 1,000 mg/day; titrate dose based on clinical response and free serum copper (non-ceruloplasmin bound copper) concentrations and/or 24-hour urinary copper excretion; usual maintenance dose: 900 to 1,500 mg/day in 2 to 3 divided doses (AASLD [Roberts 2008]; EASL 2012; ESPGHAN [Socha 2018]).
Maximum daily dose:
Children: 1,500 mg/day (AASLD [Roberts 2008]; EASL 2012; ESPGHAN [Socha 2018])
Adolescents: 2,000 mg/day
Administration
Administer at least1 hour before or 2 hours after meals and at least 1 hour apart from any drug, food, or milk. Do not open or chew capsule, swallow whole with water; any skin exposed to the contents of a capsule should be promptly washed with water.
Dietary Considerations
Should be taken 1 hour before or 2 hours after meals and at least 1 hour apart from any drug, food, or milk.
Storage
Store at 2°C to 8°C (36°F to 46°F).
Trientine Images
Drug Interactions
Carbonic Anhydrase Inhibitor Diuretics: May decrease the serum concentration of Trientine. Monitor therapy
Polyvalent Cation Containing Products: May decrease the serum concentration of Trientine. Management: Avoid concomitant administration of trientine and oral products that contain polyvalent cations. If oral iron supplements are required, separate the administration by 2 hours. If other oral polyvalent cations are needed, separate administration by 1 hour. Consider therapy modification
Adverse Reactions
Frequency not defined.
Central nervous system: Dystonia, myasthenia gravis, neurological deterioration (worsening; European Association for the Study of the Liver 2012)
Endocrine & metabolic: Iron deficiency
Gastrointestinal: Gastritis (Roberts 2008)
Hypersensitivity: Fixed drug eruption (Roberts 2008)
Neuromuscular & skeletal: Muscle spasm, systemic lupus erythematosus
<1%, postmarketing, and/or case reports: Aplastic anemia (Roberts 2008), pancytopenia (Roberts 2008), sideroblastic anemia (reversible; Roberts 2008)
Warnings/Precautions
Concerns related to adverse effects:
- Anemia: May cause iron-deficiency anemia; monitor closely, especially women.
- Copper deficiency: Induced by treatment; may lead to hepatic iron overload and/or sideroblastic anemia; reassess dose (Roberts 2008).
- Neurologic worsening: May occur with treatment initiation; less common than with penicillamine (Roberts 2008).
Other warnings/precautions:
- Hypersensitivity: Not reported with use; however, industrial workers exposed to trientine for prolonged periods have reported asthma, bronchitis, and dermatitis.
Monitoring Parameters
Periodic 24-hour urinary copper assessment (every 6 to 12 months); serum non-ceruloplasmin bound copper; LFTs; CBC; INR; urinalysis (Roberts 2008); fever and skin changes during the first month of therapy
Pregnancy
Pregnancy Considerations
Treatment of Wilson disease should be maintained during pregnancy; dose reduction (25% to 50% of prepregnancy dose) should be considered (Roberts 2008).
Patient Education
What is this drug used for?
- It is used to treat Wilson's disease.
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Lupus like rash on the cheeks or other body parts, sunburn easy, muscle or joint pain, chest pain or shortness of breath, or swelling in the arms or legs
- Difficulty moving
- Muscle spasm
- Severe loss of strength and energy
- Muscle weakness
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.