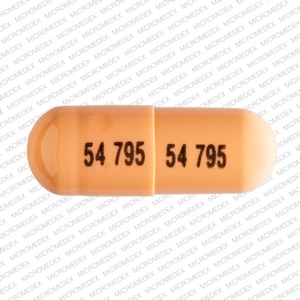
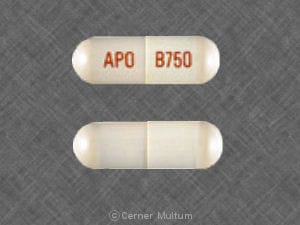
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Capsule, Oral, as disodium:
Colazal: 750 mg
Generic: 750 mg
Tablet, Oral, as disodium:
Giazo: 1.1 g [DSC]
Pharmacology
Mechanism of Action
Balsalazide is a prodrug, converted by bacterial azoreduction to 5-aminosalicylic acid (mesalamine, active), 4-aminobenzoyl-β-alanine (inert), and their metabolites. 5-aminosalicylic acid may decrease inflammation by blocking the production of arachidonic acid metabolites topically in the colon mucosa.
Pharmacokinetics/Pharmacodynamics
Absorption
Very low and variable; in children, reported systemic absorption of 5-ASA (active) lower than adults (Cmax: 67% lower, AUC: 64% lower); time to steady ~2 weeks in pediatric and adult patients
Metabolism
Azoreduced in the colon to 5-aminosalicylic acid (active), 4-aminobenzoyl-β-alanine (inert), and N-acetylated metabolites
Excretion
Feces (65% as 5-aminosalicylic acid, 4-aminobenzoyl-β-alanine, and N-acetylated metabolites); urine (<16% as N-acetylated metabolites); Parent drug: Urine or feces (<1%)
Time to Peak
Balsalazide: Capsule: 1 to 2 hours; Tablet: 0.5 hours
Half-Life Elimination
Primary effect is topical (colonic mucosa); therapeutic effect appears not to be influenced by the systemic half-life of balsalazide (1.9 hours) or its metabolites (5-ASA [9.5 hours], N-Ac-5-ASA [10.4 hours])
Protein Binding
Balsalazide: ≥99%
Use in Specific Populations
Special Populations Note
Ulcerative colitis: May have increased AUC.
Use: Labeled Indications
Ulcerative colitis: Treatment of mildly- to moderately-active ulcerative colitis
Limitations of use: Efficacy of Giazo has not been demonstrated in females.
Use: Off Label
Ulcerative colitis, remission maintenancebyes
Data from a meta-analysis of randomized, double-blind, placebo controlled trials support the use of balsalazide for 6 to 12 months for remission maintenance in ulcerative colitis Ford 2011.
Based on the American Gastroenterological Association guidelines on the management of mild to moderate ulcerative colitis, balsalazide is an option for remission maintenance in patients with extensive mild to moderate ulcerative colitis; however, evidence was low quality due to imprecision and indirectness.
Contraindications
Hypersensitivity to balsalazide or its metabolites, salicylates, or any component of the formulation
Dosage and Administration
Dosing: Adult
Note: Giazo has been discontinued in the United States for more than 1 year.
Ulcerative colitis, remission induction: Oral:
Capsule: 2.25 g 3 times daily for 8 to 12 weeks.
Tablet: Males: 3.3 g twice daily for up to 8 weeks.
Ulcerative colitis, remission maintenance (off-label use): Capsule: 1.5 or 3 g twice daily for 6 to 12 months (Ford 2011).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Note: Giazo has been discontinued in the US for >1 year.
Ulcerative colitis:
Children ≥5 years and Adolescents ≤17 years: Oral: Capsules:
2.25 g (three 750 mg capsules) 3 times daily (total daily dose: 6.75 g/day) for up to 8 weeks.
or
750 mg (one capsule) 3 times daily (total daily dose: 2.25 g/day) for up to 8 weeks.
Note: Multicenter, double-blind clinical trial in pediatric patients (n=68, ages 5 to 17 years) showed clinical improvement in both treatment groups (2.25 g/day and 6.75 g/day); there was not a statistical difference in clinical response between the two treatment groups (Quiros 2009).
Adolescents ≥18 years: Oral:
Capsule: 2.25 g (three 750 mg capsules) 3 times daily (total daily dose: 6.75 g/day) for up to 8 to 12 weeks.
Tablet: Males: 3.3 g (three 1.1 g tablets) twice daily (total daily dose: 6.6 g/day) for up to 8 weeks.
Administration
May administer with or without food.
Capsule: May be swallowed whole or may be opened and sprinkled on applesauce. Applesauce mixture may be chewed; swallow immediately, do not store mixture for later use. When sprinkled on food, may cause staining of teeth or tongue.
Dietary Considerations
Some products may contain sodium.
Storage
Store at controlled room temperature of 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Balsalazide Images
Drug Interactions
Cardiac Glycosides: 5-Aminosalicylic Acid Derivatives may decrease the serum concentration of Cardiac Glycosides. Monitor therapy
Heparin: 5-Aminosalicylic Acid Derivatives may enhance the adverse/toxic effect of Heparin. Specifically, the risk for bleeding/bruising may be increased. Monitor therapy
Heparins (Low Molecular Weight): 5-Aminosalicylic Acid Derivatives may enhance the adverse/toxic effect of Heparins (Low Molecular Weight). Specifically, the risk for bleeding/bruising may be increased. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: May enhance the nephrotoxic effect of 5-Aminosalicylic Acid Derivatives. Monitor therapy
Thiopurine Analogs: 5-Aminosalicylic Acid Derivatives may decrease the metabolism of Thiopurine Analogs. Monitor therapy
Varicella Virus-Containing Vaccines: 5-Aminosalicylic Acid Derivatives may enhance the adverse/toxic effect of Varicella Virus-Containing Vaccines. The primary concern is the potential development of Reye's Syndrome, a condition that has been associated with the use of salicylates in children with varicella infections. Consider therapy modification
Adverse Reactions
>10%:
Central nervous system: Headache (children: 15%; adults: 8%)
Gastrointestinal: Abdominal pain (children: 12% to 13%; adults: ≤6%)
1% to 10%:
Central nervous system: Fatigue (children: 4%; adults: ≤2%), insomnia (adults: 2%)
Gastrointestinal: Vomiting (children: 10%; adults: ≤4%), diarrhea (children: 9%; adults: ≤5%), exacerbation of ulcerative colitis (children: 6%; adults: 1%), nausea (adults: 5%; children: 4%), hematochezia (children: 4%), stomatitis (children: 3%), anorexia (adults: 2%), dyspepsia (adults: 2%), flatulence (adults: ≤2%), abdominal cramps (adults: 1%), constipation (adults: ≤1%), xerostomia (adults: ≤1%)
Genitourinary: Urinary tract infection (adults: 1% to 4%), dysmenorrhea (children: 3%)
Hematologic & oncologic: Anemia (4%)
Neuromuscular & skeletal: Arthralgia (adults: ≤4%), musculoskeletal pain (adults: 2%), myalgia (adults: ≤1%)
Respiratory: Pharyngitis (children: 6%; adults: 2%), flu-like symptoms (children: 4%; adults: 1%), respiratory infection (adults: ≤4%), cough (children: 3%; adults: 2%), pharyngolaryngeal pain (adults: 4%, children: 3%), rhinitis (adults: 2%)
Miscellaneous: Fever (children: 6%; adults: 2%)
<1%, postmarketing, and/or case reports: Alopecia, back pain, bowel urgency, bronchopneumonia, cholestatic jaundice, dizziness, dyspnea, edema, erythema nodosum, facial edema, fecal impaction, gastroenteritis, gastroesophageal reflux disease, hepatic cirrhosis, hepatic failure, hepatic injury, hepatic necrosis, hepatotoxicity, hyperbilirubinemia, hypersensitivity reaction, increased blood pressure, increased heart rate, increased liver enzymes, increased serum AST, interstitial nephritis, jaundice, Kawasaki-like syndrome, lethargy, malaise, myocarditis, pain, pancreatitis, pericarditis, pleural effusion, pneumonia (with and without eosinophilia), pruritus, renal failure, skin rash, vasculitis
Warnings/Precautions
Concerns related to adverse effects:
- Colitis: Symptomatic worsening of ulcerative colitis may occur following initiation of treatment.
- Intolerance syndrome: May cause an acute intolerance syndrome (cramping, acute abdominal pain, bloody diarrhea; sometimes fever, headache, rash); discontinue if this occurs.
- Staining: May cause staining of teeth or tongue if capsule is opened and sprinkled on food.
Disease-related concerns:
- Hepatic impairment: Use caution in patients with hepatic dysfunction; hepatic failure has been observed with other mesalamine (5-aminosalicylic acid) products.
- Pyloric stenosis: Use with caution in patients with pyloric stenosis; prolonged gastric retention may occur and delay release of drug in the colon.
- Renal impairment: Use with caution in patients with renal impairment; renal toxicity has been observed with other mesalamine (5-aminosalicylic acid) products.
Monitoring Parameters
Improvement or worsening of symptoms; renal function (baseline, within first 6 months of initiation, then annually thereafter) (Hanauer 2008); liver function tests (in patients with liver disease)
Pregnancy
Pregnancy Considerations
Mesalamine (5-aminosalicylic acid) is the active metabolite of balsalazide; mesalamine is known to cross the placenta. Refer to the mesalamine monograph for additional information.
Patient Education
What is this drug used for?
- It is used to treat ulcerative colitis.
Frequently reported side effects of this drug
- Headache
- Abdominal pain
- Diarrhea
- Stuffy nose
- Sore throat
- Common cold symptoms
- Joint pain
- Loss of strength and energy
- Vomiting
- Nausea
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Kidney problems like unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain
- Ulcerative colitis like severe abdominal pain or cramps; bloody stools; fever; headache; or rash
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.