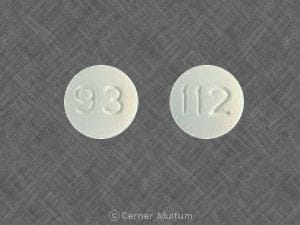
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Solution, Oral, as hydrochloride [strength expressed as base]:
Generic: 300 mg/5 mL (237 mL)
Tablet, Oral:
Cimetidine Acid Reducer: 200 mg
Generic: 200 mg, 300 mg, 400 mg, 800 mg
Pharmacology
Mechanism of Action
Competitive inhibition of histamine at H2 receptors of the gastric parietal cells resulting in reduced gastric acid secretion, gastric volume and hydrogen ion concentration reduced
Pharmacokinetics/Pharmacodynamics
Absorption
Rapid
Distribution
Children and Adolescents: 1.23 ± 0.45 L/kg (Lloyd 1985b); Adults: 1 to 1.5 L/kg (Somogyi 1983)
Metabolism
Partially hepatic, forms metabolites (Somogyi 1983)
Excretion
Primarily urine (48% as unchanged drug); feces (2%) (Somogyi 1983)
Onset of Action
1 hour
Time to Peak
Serum: Oral: 0.75 to 1.5 hours
Duration of Action
80% reduction in gastric acid secretion for 4 to 5 hours after 300 mg dose
Half-Life Elimination
Half-life elimination: Neonates: 3.6 hours (Lloyd 1985a); Children and Adolescents: 1.39 ± 0.25 hours (Lloyd 1985b); Adults: ~2 hours
Protein Binding
20% (Somogyi 1983)
Use in Specific Populations
Special Populations: Renal Function Impairment
Drug accumulation may occur in patients with severe renal failure.
Use: Labeled Indications
Duodenal ulcer: Short-term treatment of active duodenal ulcer and maintenance therapy after the healing of active ulcer.
Gastric ulcer: Short-term treatment of active, benign gastric ulcer.
Gastroesophageal reflux disease: Treatment of erosive gastroesophageal reflux disease (GERD).
Pathological hypersecretory conditions: Treatment of pathological hypersecretory conditions (eg, Zollinger-Ellison syndrome, systemic mastocytosis, multiple endocrine adenomas).
Heartburn (OTC only): Relief and prevention of heartburn associated with acid indigestion and sour stomach.
Use: Off Label
Interstitial cystitis (bladder pain syndrome)cyes
Cimetidine in the management of interstitial cystitis/bladder pain syndrome has been studied in a limited number of controlled and noncontrolled trials demonstrating moderate to complete relief of symptoms. American Urological Association guidelines recommend oral cimetidine as a second-line treatment option that may provide benefit without significant risk of adverse events; data are limited by small sample size, lack of long-term follow-up, and the potential for drug interactions.
Stress ulcer prophylaxis in critically-ill patientsyes
The American Society of Health-System Pharmacists (ASHP) recommends and supports the use of histamine H2-receptor antagonists for stress ulcer prophylaxis in critically ill patients, although there was limited data on the use of proton pump inhibitors for stress ulcer prophylaxis at the time of publication ASHP 1999.
Based on the Surviving Sepsis Campaign International Guidelines for the Management of Severe Sepsis and Septic Shock, stress ulcer prophylaxis using a histamine H2-receptor antagonist or a proton pump inhibitor is recommended in sepsis or septic shock patients who have GI bleeding risk factors.
Contraindications
Hypersensitivity to cimetidine or any component of the formulation
OTC labeling: When used for self-medication (OTC), do not use if trouble or pain when swallowing food, vomiting with blood, or bloody or black stools, allergic to cimetidine or other acid reducers. Do not use with other acid reducers.
Documentation of allergenic cross-reactivity for histamine H2 antagonists is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Dosage and Administration
Dosing: Adult
Duodenal ulcer, active: Oral: 300 mg 4 times daily or 800 mg at bedtime or 400 mg twice daily for up to 8 weeks
Note: Higher doses of 1,600 mg at bedtime for 4 weeks may be beneficial for a subpopulation of patients with larger duodenal ulcers (>1 cm defined endoscopically) who are also heavy smokers (≥1 pack/day).
Duodenal ulcer, prophylaxis: Oral: 400 mg at bedtime
Gastric ulcer, active: Oral: 300 mg 4 times daily or 800 mg at bedtime for up to 8 weeks
Gastroesophageal reflux disease: Oral: 400 mg 4 times daily or 800 mg twice daily for 12 weeks
Heartburn (OTC labeling):
Prevention: Oral: 200 mg daily up to 30 minutes prior to eating foods or beverages that cause heartburn (maximum: 400 mg per 24 hours).
Relief of symptoms: Oral: 200 mg daily; maximum: 400 mg per 24 hours.
Pathological hypersecretory conditions: Oral: 300 mg 4 times daily; adjust dose to patient response; maximum 2,400 mg/day
Interstitial cystitis (bladder pain syndrome) (off-label use): Oral: 200 mg 3 times daily or 300 to 400 mg twice daily (Dasgupta 2001; Seshadri 1994; Thilagarajah 2001)
Stress ulcer prophylaxis in critically ill patients (off-label use): Oral or NG tube: 300 mg 4 times daily (ASHP 1999). Note: Intended for patients with associated risk factors (eg, coagulopathy, mechanical ventilation for >48 hours, sepsis/septic shock); discontinue use once risk factors have resolved (Rhodes 2017).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Gastroesophageal reflux disease (GERD):
Infants, Children, and Adolescents <16 years: Limited data available: Oral: 20 to 40 mg/kg/day in 3 to 4 divided doses; maximum dose: 400 mg/dose (Cucchiara 1984; Cucchiara 1989; Lightdale 2013; NASPGHAN/ESPGHAN [Vandenplas 2009]). A pharmacokinetic modeling study suggests that the optimal dose for acid suppression is 10 mg/kg/dose every 6 hours and that some patients may need doses as high as 15 mg/kg/dose (Lambert 1992).
Adolescents ≥16 years: Oral: 400 mg 4 times daily or 800 mg twice daily for 12 weeks
Duodenal ulcer, treatment and maintenance:
Children ≥5 years and Adolescents <16 years: Limited data available, efficacy results variable: Oral: 20 to 40 mg/kg/day in 3 to 4 divided doses for 4 to 8 weeks, followed by 5 to 8 mg/kg/dose once daily at bedtime (Chiang 1989; Thomson 1983). In the largest trail, 33 patients (ages 8 to 15 years) with duodenal ulcer were allocated to receive cimetidine 20 mg/kg/day (n=8), an antacid (n=12), or sucralfate (n=17) for 8 weeks followed by maintenance therapy with once daily bedtime doses of cimetidine 5 mg/kg/dose, antacid, or sucralfate. Short-term improvement occurred in all three groups with no significant difference (Chiang 1989).
Adolescents ≥16 years: Oral: 300 mg 4 times daily or 800 mg at bedtime or 400 mg twice daily for up to 8 weeks
Duodenal ulcer, prophylaxis: Adolescents ≥16 years: Oral: 400 mg at bedtime
Gastric ulcer, active: Adolescents ≥16 years: Oral: 300 mg 4 times daily or 800 mg at bedtime for up to 8 weeks
Pathological hypersecretory conditions (eg, Zollinger-Ellison syndrome): Adolescents ≥16 years: Oral: 300 mg 4 times daily; adjust dose to patient response; maximum daily dose: 2,400 mg/day
Sour stomach/Heartburn (OTC labeling):
Prevention: Children ≥12 years and Adolescents: Oral: 200 mg daily up to 30 minutes prior to eating foods or beverages that cause heartburn (maximum daily dose: 400 mg/24 hours)
Relief of symptoms: Children ≥12 years and Adolescents: Oral: 200 mg daily; maximum daily dose: 400 mg/24 hours
Extemporaneously Prepared
Note: Commercial oral solution is available (strength expressed as base: 60 mg/mL)
A 60 mg/mL oral suspension may be made with tablets. Place twenty-four 300 mg tablets in 5 mL of sterile water for ~3-5 minutes to dissolve film coating. Crush tablets in a mortar and reduce to a fine powder. Add 10 mL of glycerin and mix to a uniform paste; mix while adding Simple Syrup, NF in incremental proportions to almost 120 mL; transfer to a calibrated bottle, rinse mortar with vehicle, and add quantity of vehicle sufficient to make 120 mL. Label "shake well" and "refrigerate". Stable for 17 days.
Nahata MC, Pai VB, and Hipple TF, Pediatric Drug Formulations, 5th ed, Cincinnati, OH: Harvey Whitney Books Co, 2004.
Administration
Administer with meals. For stress ulcer prophylaxis in critically-ill patients (off-label use), may administer via NG tube (Rhodes 2017).
Storage
Store at room temperature. Protect from light.
Cimetidine Images
Drug Interactions
Acalabrutinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Acalabrutinib. Management: To minimize the potential for a significant interaction, separate administration of these agents by giving acalabrutinib 2 hours before ingestion of a histamine-2 receptor antagonist. Consider therapy modification
Alfentanil: Cimetidine may increase the serum concentration of Alfentanil. Monitor therapy
Amiodarone: Cimetidine may increase the serum concentration of Amiodarone. Management: Consider alternatives to cimetidine. If this combination cannot be avoided, monitor for increased amiodarone concentrations/effects with cimetidine initiation/dose increase or decreased concentrations/effects with cimetidine discontinuation/dose decrease. Consider therapy modification
ARIPiprazole: CYP3A4 Inhibitors (Weak) may increase the serum concentration of ARIPiprazole. Management: Monitor for increased aripiprazole pharmacologic effects. Aripiprazole dose adjustments may or may not be required based on concomitant therapy and/or indication. Consult full interaction monograph for specific recommendations. Monitor therapy
ARIPiprazole: CYP2D6 Inhibitors (Weak) may increase the serum concentration of ARIPiprazole. Management: Monitor for increased aripiprazole pharmacologic effects. Aripiprazole dose adjustments may or may not be required based on concomitant therapy and/or indication. Consult full interaction monograph for specific recommendations. Monitor therapy
Atazanavir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Atazanavir. Management: Specific dose limitations and administration guidelines exist; consult full interaction monograph or atazanavir prescribing information. Consider therapy modification
AtorvaSTATin: May enhance the adverse/toxic effect of Cimetidine. Specifically, there is a theoretical potential for enhanced effects on reducing endogenous steroid activity. Monitor therapy
Azelastine (Systemic): Cimetidine may increase the serum concentration of Azelastine (Systemic). Monitor therapy
Bosutinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Bosutinib. Management: Administer histamine H2 receptor antagonists more than 2 hours before or after bosutinib. Consider therapy modification
Bromazepam: Cimetidine may increase the serum concentration of Bromazepam. Monitor therapy
Calcium Channel Blockers: Cimetidine may increase the serum concentration of Calcium Channel Blockers. Management: Consider alternatives to cimetidine. If no suitable alternative exists, monitor for increased effects of calcium channel blockers following cimetidine initiation/dose increase, and decreased effects following cimetidine discontinuation/dose decrease. Exceptions: AmLODIPine; Clevidipine; NiCARdipine. Consider therapy modification
CarBAMazepine: Cimetidine may increase the serum concentration of CarBAMazepine. The serum carbamazepine concentration might return to normal within one week of starting cimetidine. Monitor therapy
Carmustine: Cimetidine may enhance the myelosuppressive effect of Carmustine. Management: Consider alternatives to cimetidine in patients receiving carmustine. If the combination cannot be avoided, monitor for enhanced carmustine myelotoxicity. Consider therapy modification
Carvedilol: Cimetidine may increase the serum concentration of Carvedilol. Monitor therapy
Cefditoren: Histamine H2 Receptor Antagonists may decrease the serum concentration of Cefditoren. Management: Concomitant use of cefditoren with H2-antagonists and antacids is not recommended. Consider alternative methods to control acid reflux (eg, diet modification) or alternative antimicrobial therapy if use of H2-antagonists can not be avoided. Consider therapy modification
Cefpodoxime: Histamine H2 Receptor Antagonists may decrease the absorption of Cefpodoxime. Separate oral doses by at least 2 hours. Monitor therapy
Cefuroxime: Histamine H2 Receptor Antagonists may decrease the absorption of Cefuroxime. Separate oral doses by at least 2 hours. Avoid combination
Chlormethiazole: Cimetidine may increase the serum concentration of Chlormethiazole. Monitor therapy
Chloroquine: Cimetidine may increase the serum concentration of Chloroquine. Monitor therapy
Cisapride: Cimetidine may increase the serum concentration of Cisapride. Management: Consider alternatives to cimetidine. If this combination cannot be avoided, monitor for toxic effects of cisapride if cimetidine is initiated/dose increased, or decreased efficacy if cimetidine is discontinued/dose decreased. Consider therapy modification
Citalopram: Cimetidine may increase the serum concentration of Citalopram. Monitor therapy
CloZAPine: Cimetidine may increase the serum concentration of CloZAPine. Management: Consider use of an alternative H2 antagonist. Monitor for increased toxic effects of clozapine if cimetidine is initiated/dose increased, or decreased effects if cimetidine is discontinued/dose decreased. Consider therapy modification
Cysteamine (Systemic): Histamine H2 Receptor Antagonists may diminish the therapeutic effect of Cysteamine (Systemic). Monitor therapy
Dacomitinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Dacomitinib. Management: Administer dacomitinib at least 6 hours before or 10 hours after an histamine H2-receptor antagonist (H2RA). Consider therapy modification
Dalfampridine: Cimetidine may increase the serum concentration of Dalfampridine. Management: Recommendations differ significantly between international labelings in regards to the concomitant use of dalfampridine (referred to as fampridine in Canada) and cimetidine. Consult appropriate product labeling. Monitor therapy
Dasatinib: Histamine H2 Receptor Antagonists may decrease the absorption of Dasatinib. Management: Antacids (taken 2 hours before or after dasatinib administration) can be used in place of H2-antagonists if some acid-reducing therapy is needed. Avoid combination
Delavirdine: Histamine H2 Receptor Antagonists may decrease the serum concentration of Delavirdine. Management: Chronic therapy with H2-antagonists should be avoided in patients who are being treated with delavirdine. The clinical significance of short-term H2-antagonist therapy with delavirdine is uncertain, but such therapy should be undertaken with caution. Avoid combination
Dexmethylphenidate: Histamine H2 Receptor Antagonists may increase the absorption of Dexmethylphenidate. Specifically, H2-antagonists may interfere with the normal release of drug from the extended-release capsules (Focalin XR brand), which could result in both increased absorption (early) and decreased delayed absorption. Monitor therapy
Dofetilide: Cimetidine may increase the serum concentration of Dofetilide. This is likely via inhibition of dofetilide renal tubular secretion (primarily) and inhibition of dofetilide metabolism. Avoid combination
Doxofylline: Cimetidine may increase the serum concentration of Doxofylline. Monitor therapy
EpiRUBicin: Cimetidine may increase the serum concentration of EpiRUBicin. Avoid combination
Erdafitinib: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Erdafitinib: May increase the serum concentration of OCT2 Substrates. Monitor therapy
Erlotinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Erlotinib. Management: Avoid H2-antagonists in patients receiving erlotinib when possible. If concomitant treatment cannot be avoided, erlotinib should be dosed once daily, 10 hours after and at least 2 hours before H2-antagonist dosing. Consider therapy modification
Escitalopram: Cimetidine may increase the serum concentration of Escitalopram. Monitor therapy
Flibanserin: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Flibanserin. Monitor therapy
Flunitrazepam: Cimetidine may increase the serum concentration of Flunitrazepam. Monitor therapy
Fluorouracil Products: Cimetidine may increase the serum concentration of Fluorouracil Products. Monitor therapy
FLUoxetine: Cimetidine may increase the serum concentration of FLUoxetine. Monitor therapy
Fosamprenavir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Fosamprenavir. Cimetidine may also inhibit the metabolism of the active metabolite amprenavir, making its effects on fosamprenavir/amprenavir concentrations difficult to predict. Monitor therapy
Fosphenytoin-Phenytoin: Cimetidine may enhance the adverse/toxic effect of Fosphenytoin-Phenytoin. Cimetidine may increase the serum concentration of Fosphenytoin-Phenytoin. Management: Consider using an alternative H2-antagonist to avoid this interaction. Monitor for toxic effects of hydantoin anticonvulsants if cimetidine is initiated/dose increased. Consider therapy modification
Gefitinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Gefitinib. Management: Administer gefitinib at least 6 hours before or after administration of a histamine H2-antagonist, and closely monitor clinical response to gefitinib. Consider therapy modification
Indinavir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Indinavir. Monitor therapy
Iron Preparations: Histamine H2 Receptor Antagonists may decrease the absorption of Iron Preparations. Exceptions: Ferric Carboxymaltose; Ferric Citrate; Ferric Derisomaltose; Ferric Gluconate; Ferric Hydroxide Polymaltose Complex; Ferric Pyrophosphate Citrate; Ferumoxytol; Iron Dextran Complex; Iron Sucrose. Monitor therapy
Itraconazole: Histamine H2 Receptor Antagonists may increase the serum concentration of Itraconazole. Histamine H2 Receptor Antagonists may decrease the serum concentration of Itraconazole. Management: Administer Sporanox brand itraconazole at least 2 hours before or 2 hours after administration of any histamine H2 receptor antagonists (H2RAs). Exposure to Tolsura brand itraconazole may be increased by H2RAs; consider itraconazole dose reduction. Consider therapy modification
Ketoconazole (Systemic): Histamine H2 Receptor Antagonists may decrease the serum concentration of Ketoconazole (Systemic). Management: Administer oral ketoconazole at least 2 hours prior to use of any H2-receptor antagonist. Monitor patients closely for signs of inadequate clinical response to ketoconazole. Consider therapy modification
Lasmiditan: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Avoid combination
Ledipasvir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Ledipasvir. Consider therapy modification
Lemborexant: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Lemborexant. Management: The maximum recommended dosage of lemborexant is 5 mg, no more than once per night, when coadministered with weak CYP3A4 inhibitors. Consider therapy modification
Lomitapide: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Lomitapide. Management: Patients on lomitapide 5 mg/day may continue that dose. Patients taking lomitapide 10 mg/day or more should decrease the lomitapide dose by half. The lomitapide dose may then be titrated up to a max adult dose of 30 mg/day. Consider therapy modification
Lumacaftor and Ivacaftor: May decrease the serum concentration of P-glycoprotein/ABCB1 Substrates. Lumacaftor and Ivacaftor may increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Mebendazole: Cimetidine may increase the serum concentration of Mebendazole. Monitor therapy
Melatonin: Cimetidine may increase the serum concentration of Melatonin. Monitor therapy
Meperidine: Cimetidine may increase the serum concentration of Meperidine. Monitor therapy
Mesalamine: Histamine H2 Receptor Antagonists may diminish the therapeutic effect of Mesalamine. Histamine H2-Antagonist-mediated increases in gastrointestinal pH may cause the premature release of mesalamine from specific sustained-release mesalamine products. Management: Consider avoiding concurrent administration of high-dose histamine H2-receptor antagonists with sustained-release mesalamine products. Consider therapy modification
MetFORMIN: Cimetidine may increase the serum concentration of MetFORMIN. Management: Consider alternatives to cimetidine in patients receiving metformin due to a potential for increased metformin concentrations and toxicity (including lactic acidosis). Consider therapy modification
Methylphenidate: Histamine H2 Receptor Antagonists may increase the absorption of Methylphenidate. Specifically, H2-antagonists may interfere with the normal release of drug from the extended-release capsules (Ritalin LA brand), which could result in both increased absorption (early) and decreased delayed absorption. Monitor therapy
Mirtazapine: Cimetidine may increase the serum concentration of Mirtazapine. Monitor therapy
Moclobemide: Cimetidine may decrease the metabolism of Moclobemide. Management: Consider using alternative agents to lower gastric pH in order to avoid this interaction. If combined, a moclobemide dose reduction of 50% is recommended and patients should be monitored for increased moclobemide effects/toxicities. Consider therapy modification
Multivitamins/Minerals (with ADEK, Folate, Iron): Histamine H2 Receptor Antagonists may decrease the serum concentration of Multivitamins/Minerals (with ADEK, Folate, Iron). Specifically, the absorption of iron may be impaired by H2-antagonists. Monitor therapy
Nelfinavir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Nelfinavir. Concentrations of the active M8 metabolite may also be reduced. Monitor therapy
Neratinib: Cimetidine may increase the serum concentration of Neratinib. Cimetidine may decrease the serum concentration of Neratinib. Specifically, cimetidine may reduce neratinib absorption. Management: Avoid concomitant use of neratinib and cimetidine. Avoid combination
Nicotine: Cimetidine may increase the serum concentration of Nicotine. Monitor therapy
Nilotinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Nilotinib. Management: The nilotinib dose should be given 10 hours after or 2 hours before the H2 receptor antagonist in order to minimize the risk of a significant interaction. Consider therapy modification
Nitisinone: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
PARoxetine: Cimetidine may increase the serum concentration of PARoxetine. Monitor therapy
PAZOPanib: Histamine H2 Receptor Antagonists may decrease the serum concentration of PAZOPanib. Management: Avoid the use of histamine H2-antagonists in combination with pazopanib. Strategies to minimize the expected interaction between these agents (eg, dose separation) have not been investigated. Avoid combination
Pentoxifylline: Cimetidine may increase the serum concentration of Pentoxifylline. Monitor therapy
Perhexiline: CYP2D6 Inhibitors (Weak) may increase the serum concentration of Perhexiline. Monitor therapy
Pexidartinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Pexidartinib. Management: Administer pexidartinib 2 hours before or 10 hours after histamine H2 receptor antagonists. Consider therapy modification
P-glycoprotein/ABCB1 Inducers: May decrease the serum concentration of P-glycoprotein/ABCB1 Substrates. P-glycoprotein inducers may also further limit the distribution of p-glycoprotein substrates to specific cells/tissues/organs where p-glycoprotein is present in large amounts (e.g., brain, T-lymphocytes, testes, etc.). Monitor therapy
P-glycoprotein/ABCB1 Inhibitors: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. P-glycoprotein inhibitors may also enhance the distribution of p-glycoprotein substrates to specific cells/tissues/organs where p-glycoprotein is present in large amounts (e.g., brain, T-lymphocytes, testes, etc.). Monitor therapy
Pilsicainide: Cimetidine may increase the serum concentration of Pilsicainide. Monitor therapy
Pimozide: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Pimozide. Avoid combination
Posaconazole: Histamine H2 Receptor Antagonists may decrease the serum concentration of Posaconazole. Management: Avoid concurrent use of oral suspension with H2-antagonists whenever possible. Monitor patients closely for decreased antifungal effects if this combination is used. Delayed-release posaconazole tablets may be less likely to interact. Consider therapy modification
Pramipexole: Cimetidine may increase the serum concentration of Pramipexole. Monitor therapy
Praziquantel: Cimetidine may increase the serum concentration of Praziquantel. Monitor therapy
Pretomanid: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Procainamide: Cimetidine may increase the serum concentration of Procainamide. Management: Consider an alternative H2-receptor antagonist in patients taking procainamide. If combined, monitor for increased therapeutic effects/toxicity of procainamide. Consider therapy modification
Propafenone: Cimetidine may increase the serum concentration of Propafenone. Monitor therapy
QuiNIDine: Cimetidine may increase the serum concentration of QuiNIDine. Management: Consider alternatives to cimetidine. If the combination cannot be avoided, monitor for increased quinidine concentrations/toxicity with cimetidine initiation/dose increase, or decreased concentrations/effects with cimetidine discontinuation/dose decrease. Consider therapy modification
QuiNINE: Cimetidine may increase the serum concentration of QuiNINE. Management: Consider using an alternative H2-receptor antagonist (eg, ranitidine) instead of cimetidine due to a lower interaction risk. If combined, monitor patients closely for signs and symptoms of quinine toxicity. Consider therapy modification
Ranolazine: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Rilpivirine: Histamine H2 Receptor Antagonists may decrease the serum concentration of Rilpivirine. Management: Administer histamine H2 receptor antagonists at least 12 hours before or 4 hours after rilpivirine. Consider therapy modification
Risedronate: Histamine H2 Receptor Antagonists may increase the serum concentration of Risedronate. This applies specifically to delayed-release risedronate. Avoid combination
Roflumilast: Cimetidine may increase serum concentrations of the active metabolite(s) of Roflumilast. Cimetidine may increase the serum concentration of Roflumilast. Monitor therapy
Saquinavir: Histamine H2 Receptor Antagonists may increase the serum concentration of Saquinavir. Monitor therapy
Secretin: Histamine H2 Receptor Antagonists may diminish the diagnostic effect of Secretin. Specifically, use of H2-Antagonists may cause a hyperresponse in gastrin secretion in response to secretin stimulation testing, falsely suggesting gastrinoma. Management: Avoid concomitant use of histamine H2-antagonists (H2RAs) and secretin. Discontinue H2RAs at least 2 days prior to secretin administration. Consider therapy modification
Sulfonylureas: Cimetidine may increase the serum concentration of Sulfonylureas. Monitor therapy
Tafenoquine: May increase the serum concentration of OCT2 Substrates. Management: Avoid use of OCT2 substrates with tafenoquine, and if the combination cannot be avoided, monitor closely for evidence of toxicity of the OCT2 substrate and consider a reduced dose of the OCT2 substrate according to that substrate's labeling. Consider therapy modification
Tamsulosin: Cimetidine may increase the serum concentration of Tamsulosin. Monitor therapy
Teriflunomide: May increase the serum concentration of OAT1/3 Substrates. Monitor therapy
Theophylline Derivatives: CYP1A2 Inhibitors (Weak) may increase the serum concentration of Theophylline Derivatives. Exceptions: Dyphylline. Monitor therapy
TiZANidine: CYP1A2 Inhibitors (Weak) may increase the serum concentration of TiZANidine. Management: Avoid these combinations when possible. If combined use is necessary, initiate tizanidine at an adult dose of 2 mg and increase in 2 to 4 mg increments based on patient response. Monitor for increased effects of tizanidine, including adverse reactions. Consider therapy modification
Tolvaptan: May increase the serum concentration of OAT1/3 Substrates. Management: Patients being treated with the Jynarque brand of tolvaptan should avoid concomitant use of OAT1/3 substrates. Concentrations and effects of the OAT1/3 substrate would be expected to increase with any combined use. Consider therapy modification
Triazolam: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Triazolam. Management: Consider triazolam dose reduction in patients receiving concomitant weak CYP3A4 inhibitors. Consider therapy modification
Tricyclic Antidepressants: Cimetidine may decrease the metabolism of Tricyclic Antidepressants. Monitor therapy
Ubrogepant: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Ubrogepant. Management: In patients taking weak CYP3A4 inhibitors, the initial and second dose (if needed) of ubrogepant should be limited to 50 mg. Consider therapy modification
Urapidil: Cimetidine may enhance the hypotensive effect of Urapidil. Monitor therapy
Varenicline: Histamine H2 Receptor Antagonists may increase the serum concentration of Varenicline. Management: Monitor for increased varenicline adverse effects with concomitant use of cimetidine or other H2-antagonists, particularly in patients with severe renal impairment. International product labeling recommendations vary. Consult appropriate labeling. Monitor therapy
Velpatasvir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Velpatasvir. Monitor therapy
Vitamin K Antagonists (eg, warfarin): Cimetidine may enhance the anticoagulant effect of Vitamin K Antagonists. Consider therapy modification
Zaleplon: Cimetidine may increase the serum concentration of Zaleplon. Management: The initial dose of zaleplon should be limited to 5 mg in patients taking cimetidine. Monitor patients for increased zaleplon effects/toxicities (ie, sedation, CNS depression) when these agents are combined. Consider therapy modification
ZOLMitriptan: Cimetidine may increase the serum concentration of ZOLMitriptan. Management: Limit the maximum single dose of zolmitriptan to 2.5 mg, not to exceed 5 mg in 24 hours, when coadministered with cimetidine. Consider therapy modification
Adverse Reactions
1% to 10%:
Central nervous system: Headache (2% to 4%), dizziness (1%), drowsiness (1%)
Endocrine & metabolic: Gynecomastia (≤4%)
Gastrointestinal: Diarrhea (1%)
Frequency not defined: Renal: Increased serum creatinine
Postmarketing: Agitation, agranulocytosis, alopecia, anaphylaxis, anxiety, aplastic anemia, arthralgia, confusion, decreased white blood cell count, depression, disorientation, fever (Potter 1986), hallucination, hemolytic anemia (immune-based), hepatic fibrosis, hypersensitivity angiitis, hypersensitivity reaction, hypotension, impotence (Jensen 1983), increased serum transaminases, interstitial nephritis, myalgia, pancreatitis, pancytopenia, polymyositis (Watson 1983), psychosis, urinary retention, thrombocytopenia
Warnings/Precautions
Concerns related to adverse effects:
- Confusion: Rare cases of reversible confusion have been associated with cimetidine; usually in elderly or severely ill patients, or in patients with renal or hepatic impairment.
- Vitamin B12 deficiency: Prolonged treatment (≥2 years) may lead to vitamin B12 malabsorption and subsequent vitamin B12 deficiency. The magnitude of the deficiency is dose-related and the association is stronger in females and those younger in age (<30 years); prevalence is decreased after discontinuation of therapy (Lam 2013).
Disease-related concerns:
- Gastric malignancy: Relief of symptoms does not preclude the presence of a gastric malignancy.
- Hepatic impairment: Use with caution in patients with hepatic impairment.
- Renal impairment: Use with caution in patients with renal impairment; dosage adjustment recommended.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Elderly: Use caution in this age group due to risk of confusion and other CNS effects. Cimetidine should be avoided in those older adults with, or at risk of, delirium.
- Immunocompromised patients: May have increased risk of hyperinfection of strongyloidiasis.
- Pediatric: Use of gastric acid inhibitors, including proton pump inhibitors and H2 blockers, has been associated with an increased risk for development of acute gastroenteritis and community-acquired pneumonia in pediatric patients (Canani 2006).
Other warnings/precautions:
- Serum creatinine/creatinine clearance estimates: Cimetidine can cause a transient and reversible rise in serum creatinine and/or decrease in creatinine clearance (when using routine creatinine clearance estimation formulas). This interference is likely due to competitive inhibition of cimetidine with creatinine for active tubular secretion and independent of an actual change in glomerular filtration rate (GFR). In patients with normal renal function, the rise in serum creatinine may not be clinically apparent, but in patients with varying degrees of renal impairment, this rise in serum creatinine may be more significant. Thus, treatment with cimetidine in patients with renal failure may invalidate measurements of serum creatinine and estimates of creatinine clearance (Andreev 1999; Larsson 1980). However, creatinine clearance estimation formulas, such as Cockcroft-Gault, are known to overestimate actual GFR, particularly in patients with renal impairment, and use of cimetidine has been explored clinically to improve the accuracy of creatinine clearance estimates in these patients (Choi 1993; Van Acker 1992).
- OTC labeling: When used for self-medication (OTC), notify health care provider before use if any of the following are present: Frequent chest pain; frequent wheezing particularly with heartburn; nausea/vomiting; unexplained weight loss; stomach pain; heartburn >3 months; heartburn with light-headedness, sweating, or dizziness; chest pain or shoulder pain with shortness of breath; sweating or pain that spreads to arms, neck, or shoulders; light-headedness. Stop use and notify health care provider if heartburn continues or worsens, stomach pain continues, or if use is required >14 days.
Monitoring Parameters
CBC, gastric pH; monitor renal function to correct dose; occult blood with GI bleeding; signs of confusion.
Pregnancy
Pregnancy Risk Factor
B
Pregnancy Considerations
Adverse events have not been observed in animal reproduction studies. Cimetidine crosses the placenta (Howe 1981). Histamine H2 antagonists have been evaluated for the treatment of gastroesophageal reflux disease (GERD), as well as gastric and duodenal ulcers during pregnancy (Cappell 2003; Richter 2003). Histamine H2 antagonists may be used for aspiration prophylaxis prior to cesarean delivery (ASA 2007).
Patient Education
What is this drug used for?
- It is used to treat or prevent GI (gastrointestinal) ulcers.
- It is used to treat gastroesophageal reflux disease (GERD; acid reflux).
- It is used to treat heartburn and sour stomach.
- It is used to treat syndromes caused by lots of stomach acid.
- It may be given to you for other reasons. Talk with the doctor.
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Agitation
- Confusion
- Mood changes
- Sensing things that seem real but are not
- Enlarged breasts
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.