Boxed Warning
Experienced physician (injection only):
Cladribine should be administered under the supervision of a qualified health care provider experienced in the use of antineoplastic therapy.
Bone marrow suppression (injection only):
Suppression of bone marrow function should be anticipated. This is usually reversible and appears to be dose dependent.
Malignancy (oral tablet only):
Treatment with cladribine oral tablets may increase the risk of malignancy. Cladribine oral tablets are contraindicated in patients with current malignancy. In patients with prior malignancy or with increased risk of malignancy, evaluate the benefits and risks of the use of cladribine oral tablets on an individual patient basis. Follow standard cancer screening guidelines in patients treated with cladribine oral tablets.
Neurotoxicity (injection only):
Serious neurological toxicity (including irreversible paraparesis and quadriparesis) has been reported in patients who received cladribine by continuous infusion at high doses (4 to 9 times the recommended dose for hairy cell leukemia). Neurologic toxicity appears to demonstrate a dose relationship; however, severe neurological toxicity has been reported rarely following treatment with standard cladribine dosing regimens.
Renal toxicity (injection only):
Acute nephrotoxicity has been observed with high doses of cladribine (4 to 9 times the recommended dose for hairy cell leukemia), especially when given concomitantly with other nephrotoxic agents/therapies.
Risk of teratogenicity (oral tablet only):
Cladribine oral tablets are contraindicated for use in pregnant women and in women and men of reproductive potential who do not plan to use effective contraception because of the potential for fetal harm. Malformations and embryolethality occurred in animals. Exclude pregnancy before the start of treatment with cladribine oral tablets in females of reproductive potential. Advise females and males of reproductive potential to use effective contraception during cladribine oral tablets dosing and for 6 months after the last dose in each treatment course. Stop cladribine oral tablets if the patient becomes pregnant.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Solution, Intravenous [preservative free]:
Generic: 10 mg/10 mL (10 mL)
Tablet Therapy Pack, Oral:
Mavenclad (10 Tabs): 10 mg [five packs containing 2 tablets ea] (10 ea)
Mavenclad (4 Tabs): 10 mg [four packs containing 1 tablet] (4 ea)
Mavenclad (5 Tabs): 10 mg [five packs containing 1 tablet] (5 ea)
Mavenclad (6 Tabs): 10 mg [one pack containing 2 tablets; four packs containing 1 tablet] (6 ea)
Mavenclad (7 Tabs): 10 mg [two packs containing 2 tablets ea; three packs containing 1 tablet ea] (7 ea)
Mavenclad (8 Tabs): 10 mg [three packs containing 2 tablets ea; two packs containing 1 tablet ea] (8 ea)
Mavenclad (9 Tabs): 10 mg [four packs containing 2 tablets ea; one pack containing 1 tablet] (9 ea)
Pharmacology
Mechanism of Action
Cladribine is a purine nucleoside analogue; it is a prodrug which is activated by phosphorylation and converted into the active moiety, Cd-ATP. This active form incorporates into DNA to result in the breakage of DNA strand and shutdown of DNA synthesis and repair. This also results in a depletion of nicotinamide adenine dinucleotide and adenosine triphosphate (ATP). Cladribine is cell-cycle nonspecific. The mechanism of cladribine in treating multiple sclerosis (MS) is unknown, but may involve cytotoxic effects on B and T lymphocytes that result from the shutdown of DNA synthesis, leading to a depletion of lymphocytes.
Pharmacokinetics/Pharmacodynamics
Absorption
Oral: Rapid; delayed with food
Distribution
Vd: Children 8 months to 18 years: 12.7 ± 8.5 L/kg; penetrates CSF (CSF concentrations are ~18% of plasma concentration) (Kearns 1994); Adults: 480 to 490 L or ~9 L/kg; penetrates CSF (CSF concentrations are ~25% of plasma concentrations)
Metabolism
Prodrug; activated via phosphorylation to active metabolite, Cd-ATP; negligible hepatic metabolism.
Excretion
Urine (18% to 28.5%)
Time to Peak
Oral: Median 0.5 hour (range 0.5 to 1.5 hours) (fasting); 1.5 hours (range 1 to 3 hours) (with high-fat meal)
Half-Life Elimination
Children 8 months to 18 years: IV: 19.7 ± 3.4 hours (Kearns 1994); Adults: After a 2-hour infusion (with normal renal function): 5.4 hours; Oral: ~24 hours
Protein Binding
~20%
Use in Specific Populations
Special Populations: Renal Function Impairment
Mild impairment (CrCl 60 to 90 mL/minute): 18% decrease in total clearance; 25% increase in cladribine exposure
Moderate or serious impairment: Limited clinical experience.
Use: Labeled Indications
Hairy cell leukemia (injection only): Treatment of active hairy cell leukemia as defined by clinically significant anemia, neutropenia, thrombocytopenia, or disease-related symptoms.
Multiple sclerosis, relapsing (oral tablet only): Treatment of relapsing forms of multiple sclerosis (MS), including relapsing-remitting (RRMS) and active secondary progressive disease in adults who have had inadequate response or are intolerant to other therapies for multiple sclerosis.
Limitations of use: Not recommended for patients with clinically isolated syndrome.
Use: Off Label
Acute myeloid leukemiaa
Data from a large multicenter randomized phase III trial support the use of cladribine (in combination with daunorubicin and cytarabine) as induction therapy in newly diagnosed adult patients with acute myeloid leukemia (AML) Holowiecki 2012. In addition, data from studies in adult patients with relapsed or refractory AML supports the use of cladribine (in combination with cytarabine and G-CSF [CLAG regimen] or with cytarabine, mitoxantrone, and G-CSF [CLAG-M regimen]) in the treatment of refractory or relapsed AML in adults Robak 2000, Wierzbowska 2008, Wrzesien-Kus 2003.
Mantle cell lymphomab
Data from 2 prospective, open label studies in patients with either untreated or relapsed mantle cell lymphoma support the use of cladribine in the treatment of mantle cell lymphoma Rummel 1999, Inwards 2008.
T-cell large granular lymphocytic leukemia (refractory)c
Data from a single case report suggest that cladribine may have efficacy in the management of T-cell large granular lymphocytic leukemia Edelman 1997.
Waldenström macroglobulinemiab
Data from one single-center nonrandomized clinical trial and one multi-center phase II clinical trial in patients with Waldenström macroglobulinemia support the use of cladribine (either alone or in combination with rituximab) for the treatment of Waldenström macroglobulinemia Dimopoulos 1994, Laszlo 2010.
Contraindications
Hypersensitivity to cladribine or any component of the formulation
Oral tablet: Current malignancy; pregnancy; men or women of reproductive potential who do not plan to use effective contraception during therapy and for 6 months after the last dose in each treatment course; HIV infection; active chronic infections (eg, hepatitis or tuberculosis); breastfeeding (during treatment or for 10 days after last dose)
Oral tablet [Canadian product]: Additional contraindications (not in US labeling): Increased risk of opportunistic infections (including those immunocompromised due to therapy [immunosuppressive or immunomodulating, antineoplastic or myelosuppressive therapies; total lymphoid irradiation; bone marrow transplantation] or disease [immunodeficiency syndrome]); history of progressive multifocal leukoencephalopathy; moderate or severe renal impairment (CrCL <60 mL/minute)
Dosage and Administration
Dosing: Adult
Acute myeloid leukemia (newly diagnosed), induction (off-label use): DAC regimen: IV: 5 mg/m2 over 3 hours on days 1 to 5 (in combination with daunorubicin and cytarabine); a second induction cycle may be administered if needed (Holowiecki 2012).
Acute myeloid leukemia (relapsed/refractory), induction (off-label use): CLAG or CLAG-M regimen: IV: 5 mg/m2/day over 2 hours for 5 days (in combination with cytarabine and filgrastim ± mitoxantrone); a second induction cycle may be administered if needed (Robak 2000; Wierzbowska 2008; Wrzesien-Kus 2003).
Hairy cell leukemia:
IV: 0.14 mg/kg/day over 2 hours for 5 days (Grever 2017) or 0.1 mg/kg/day continuous infusion for 7 days for 1 cycle (Goodman 2003; Saven 1998) or 0.09 mg/kg/day continuous infusion for 7 days for 1 cycle (Cladribine labeling [Fresenius] August 2016).
SubQ (off-label route): 0.1 to 0.14 mg/kg/day for 5 days (Grever 2017).
Mantle cell lymphoma (off-label use): IV: 5 mg/m2/day over 2 hours for 5 days every 4 weeks for 2 to 6 cycles (Inwards 2008) or 5 mg/m2/day over 2 hours for 5 days every 4 to 5 weeks for a maximum of 6 cycles (Rummel 1999) or 5 mg/m2/day over 2 hours for 5 days every 4 weeks for 2 to 6 cycles (in combination with rituximab) (Inwards 2008).
Multiple sclerosis, relapsing: Oral: 3.5 mg/kg over 2-year treatment course, administered as 1.75 mg/kg in each year. Divide the 1.75 mg/kg dose over 2 cycles, each cycle lasting 4 to 5 consecutive days; do not administer more than 20 mg/day. In the first-year treatment course, initiate the first cycle at any time; administer the second cycle 23 to 27 days after the last dose of the first cycle. In the second-year treatment course, initiate the first cycle ≥43 weeks after the last dose of the first year's second cycle. Administer the second cycle 23 to 27 days after the last dose of the second year's first cycle. Following 2 years of treatment, do not administer oral cladribine during the next 2 years. Refer to manufacturer's labeling for additional dosing details, including dosing tables.
Note: Lymphocytes must be within normal limits before initiating first treatment course and ≥800 cells/mm3 before initiating the second treatment course. If needed, the second treatment course can be delayed up to 6 months to allow lymphocytes to recover to ≥800 cells/mm3. If lymphocytes <800 cells/mm3 after 6-month delay, do not continue cladribine.
Missed doses: If a dose is not administered on a scheduled day, administer the missed dose on the following day and extend the number of days in that treatment cycle. If 2 consecutive doses are missed, extended the treatment cycle by 2 days.
T-cell large granular lymphocytic leukemia, refractory (off-label use; based on limited data): IV: 0.1 mg/kg/day continuous infusion for 7 days for 2 cycles (Edelman 1997).
Waldenström macroglobulinemia (off-label use):
IV: 0.1 mg/kg/day continuous infusion for 7 days every 4 weeks for 2 cycles (Dimopoulos 1994).
SubQ: 0.1 mg/kg/day for 5 consecutive days every month for 4 cycles (in combination with rituximab) (Laszlo 2010).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Note: Dosing and frequency may vary by protocol and/or treatment phase; refer to specific protocol.
Acute myeloid leukemia: Limited data available: Infants, Children, and Adolescents: IV: 8.9 mg/m2/day continuous infusion for 5 days for 1 or 2 courses (Krance 2001) or 9 mg/m2/day over 30 minutes for 5 days for 1 course (in combination with cytarabine) (Crews 2002; Rubnitz 2009)
Langerhans cell histiocytosis, refractory: Limited data available: Infants, Children, and Adolescents: IV: 5 mg/m2/day over 2 hours for 5 days every 21 days for up to 6 cycles (Weitzman 2009); has also been administered as a continuous IV infusion: 5 mg/m2/day over 24 hours for 3 days; if tolerated, dose increased to 6.5 mg/m2/day over 24 hours for 3 days for subsequent courses (Stine 2004)
Dosing: Obesity
IV: ASCO Guidelines for appropriate chemotherapy dosing in obese adults with cancer (excluding leukemias): Utilize patient's actual body weight (full weight) for calculation of body surface area- or weight-based dosing, particularly when the intent of therapy is curative; manage regimen-related toxicities in the same manner as for nonobese patients; if a dose reduction is utilized due to toxicity, consider resumption of full weight-based dosing with subsequent cycles, especially if cause of toxicity (eg, hepatic or renal impairment) is resolved (Griggs 2012).
Reconstitution
A precipitate may develop at low temperatures and may be resolubilized at room temperature or by shaking the solution vigorously. Inadvertent freezing does not affect the solution; if freezing occurs prior to dilution, allow to thaw naturally; do not heat or microwave; do not refreeze.
Do not use D5W as a diluent due to increased degradation of cladribine. Prepare cladribine infusions with NS.
To prepare a 24-hour continuous infusion: Dilute in 500 mL NS. The manufacturer recommends filtering with a 0.22 micron hydrophilic syringe filter prior to adding to infusion bag.
To prepare a 7-day continuous infusion: Dilute to a total volume of 100 mL in a CADD medication cassette reservoir using bacteriostatic NS. Filter diluent and cladribine with a 0.22 micron hydrophilic filter prior to adding to cassette/reservoir.
Administration
IV: Usually administered as a continuous infusion or over 2 hours; infusions over 3 hours have also been reported; refer to specific reference for infusion rate.
SubQ (off-label route): May also be administered SubQ (Grever 2017; Laszlo 2010)
Oral: Administer with water (with or without food); swallow whole immediately after removing from packaging; do not chew. Patients should use dry hands for handling and avoid prolonged contact with skin; wash hands and any surface that came in contact with the tablet thoroughly afterwards. Separate administration from any other oral medication by 3 hours. Refer to manufacturer's labeling for additional administration detail.
Storage
Store intact vials refrigerated at 2°C to 8°C (36°F to 46°F). Protect from light. A precipitate may develop at low temperatures and may be resolubilized at room temperature or by shaking the solution vigorously. Inadvertent freezing does not affect the solution; if freezing occurs prior to dilution, allow to thaw naturally prior to reconstitution; do not heat or microwave; do not refreeze.
Solutions for infusion diluted in NS in PVC containers are stable for 24 hours at room temperature and normal lighting conditions.
24-hour continuous infusion: Dilutions in NS for infusion should be used promptly; if not used promptly, the 24-hour infusion may be stored refrigerated for up to 8 hours prior to administration.
7-day continuous infusion: Dilutions in NS for infusion should be used promptly; if not used promptly, the 7-day infusion may be stored refrigerated for up to 8 hours prior to administration. Reconstituted solution is stable for 7 days (when diluted in bacteriostatic NS) in a CADD medication cassette reservoir. For patients weighing >85 kg, the effectiveness of the preservative in the bacteriostatic diluent may be reduced (due to dilution).
Oral tablet: Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Protect from moisture. Store in original packing until immediately before use.
Drug Interactions
Agents that Undergo Intracellular Phosphorylation: May diminish the therapeutic effect of Cladribine. Avoid combination
BCG (Intravesical): Immunosuppressants may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
BCG (Intravesical): Myelosuppressive Agents may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
BCRP/ABCG2 Inducers: May decrease the serum concentration of Cladribine. Monitor therapy
BCRP/ABCG2 Inhibitors: May increase the serum concentration of Cladribine. Management: Avoid concomitant use of BCRP inhibitors during the 4 to 5 day oral cladribine treatment cycles whenever possible. If combined, consider dose reduction of the BCRP inhibitor and separation in the timing of administration. Consider therapy modification
CloZAPine: Myelosuppressive Agents may enhance the adverse/toxic effect of CloZAPine. Specifically, the risk for neutropenia may be increased. Monitor therapy
Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Monitor therapy
Deferiprone: Myelosuppressive Agents may enhance the neutropenic effect of Deferiprone. Management: Avoid the concomitant use of deferiprone and myelosuppressive agents whenever possible. If this combination cannot be avoided, monitor the absolute neutrophil count more closely. Consider therapy modification
Denosumab: May enhance the adverse/toxic effect of Immunosuppressants. Specifically, the risk for serious infections may be increased. Monitor therapy
Dipyrone: May enhance the adverse/toxic effect of Myelosuppressive Agents. Specifically, the risk for agranulocytosis and pancytopenia may be increased Avoid combination
Echinacea: May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification
Hormonal Contraceptives: Cladribine may diminish the therapeutic effect of Hormonal Contraceptives. Management: Women using systemically acting hormonal contraceptives should add a barrier method during cladribine dosing and for at least 4 weeks after the last dose in each treatment course. Consider therapy modification
Immunosuppressants: Cladribine may enhance the immunosuppressive effect of Immunosuppressants. Avoid combination
Inhibitors of Equilibrative Nucleoside (ENT1) and Concentrative Nucleoside (CNT3) Transport Proteins: May increase the serum concentration of Cladribine. Management: Avoid concomitant use of ENT1 or CNT3 inhibitors during the 4 to 5 day oral cladribine treatment cycles whenever possible. If combined, consider an ENT1 or CNT3 inhibitor dose reduction and separation in the timing of administration. Consider therapy modification
Interferons (Beta): Cladribine may enhance the adverse/toxic effect of Interferons (Beta). Specifically, the risk for lymphopenia may be increased. Avoid combination
Lenograstim: Antineoplastic Agents may diminish the therapeutic effect of Lenograstim. Management: Avoid the use of lenograstim 24 hours before until 24 hours after the completion of myelosuppressive cytotoxic chemotherapy. Consider therapy modification
Lipegfilgrastim: Antineoplastic Agents may diminish the therapeutic effect of Lipegfilgrastim. Management: Avoid concomitant use of lipegfilgrastim and myelosuppressive cytotoxic chemotherapy. Lipegfilgrastim should be administered at least 24 hours after the completion of myelosuppressive cytotoxic chemotherapy. Consider therapy modification
Mesalamine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Myelosuppressive Agents: Cladribine may enhance the myelosuppressive effect of Myelosuppressive Agents. Avoid combination
Natalizumab: Immunosuppressants may enhance the adverse/toxic effect of Natalizumab. Specifically, the risk of concurrent infection may be increased. Avoid combination
Nivolumab: Immunosuppressants may diminish the therapeutic effect of Nivolumab. Consider therapy modification
Palifermin: May enhance the adverse/toxic effect of Antineoplastic Agents. Specifically, the duration and severity of oral mucositis may be increased. Management: Do not administer palifermin within 24 hours before, during infusion of, or within 24 hours after administration of myelotoxic chemotherapy. Consider therapy modification
P-glycoprotein/ABCB1 Inducers: May decrease the serum concentration of Cladribine. Monitor therapy
Pidotimod: Immunosuppressants may diminish the therapeutic effect of Pidotimod. Monitor therapy
Pimecrolimus: May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Promazine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Roflumilast: May enhance the immunosuppressive effect of Immunosuppressants. Consider therapy modification
Sipuleucel-T: Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Management: Evaluate patients to see if it is medically appropriate to reduce or discontinue therapy with immunosuppressants prior to initiating sipuleucel-T therapy. Consider therapy modification
Smallpox and Monkeypox Vaccine (Live): Immunosuppressants may diminish the therapeutic effect of Smallpox and Monkeypox Vaccine (Live). Monitor therapy
Tacrolimus (Topical): May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide. Monitor therapy
Trastuzumab: May enhance the neutropenic effect of Immunosuppressants. Monitor therapy
Upadacitinib: Immunosuppressants may enhance the immunosuppressive effect of Upadacitinib. Avoid combination
Vaccines (Inactivated): Immunosuppressants may diminish the therapeutic effect of Vaccines (Inactivated). Management: Vaccine efficacy may be reduced. Complete all age-appropriate vaccinations at least 2 weeks prior to starting an immunosuppressant. If vaccinated during immunosuppressant therapy, revaccinate at least 3 months after immunosuppressant discontinuation. Consider therapy modification
Vaccines (Live): Immunosuppressants may enhance the adverse/toxic effect of Vaccines (Live). Immunosuppressants may diminish the therapeutic effect of Vaccines (Live). Management: Avoid use of live organism vaccines with immunosuppressants; live-attenuated vaccines should not be given for at least 3 months after immunosuppressants. Exceptions: Smallpox and Monkeypox Vaccine (Live). Avoid combination
Adverse Reactions
>10%:
Central nervous system: Fatigue (IV: 31%), headache (PO: 25%; IV: 14%), high fever (IV: 11%)
Dermatologic: Skin rash (IV: 16%; PO: <1%)
Gastrointestinal: Nausea (IV: 22%; PO: 10%)
Hematologic & oncologic: Lymphocytopenia (24% to 87%), severe neutropenia (IV: 70%), severe anemia (IV: 37%), bone marrow depression (34%), decreased hemoglobin (oral: 26%), thrombocytopenia (11% to 12%)
Hypersensitivity: Hypersensitivity reaction (11%)
Infection: Infection (PO: 49%; IV: 6% to 28%), bacterial infection (IV: 12%)
Local: Injection site reaction (IV: 11%)
Respiratory: Upper respiratory tract infection (PO: 38%)
Miscellaneous: Fever (IV: 33% to 69%; PO: 5%)
1% to 10%:
Cardiovascular: Hypertension (PO: 5%), peripheral edema (IV: 2%), tachycardia (IV: 2%)
Central nervous system: Dizziness (IV: 6%), pain (IV: 6%), insomnia (3% to 6%), depression (PO: 5%), malaise (IV: 5%), chills (IV: 2%), anxiety (IV: 1%), myasthenia (IV: 1%)
Dermatologic: Alopecia (PO: 3%), hyperhidrosis (IV: 3%), ecchymosis (IV: 2%), pruritus (IV: 2%)
Gastrointestinal: Vomiting (IV: 9%), decreased appetite (IV: 8%), diarrhea (IV: 7%), abdominal pain (IV: 4%), constipation (IV: 4%), oral herpes simplex infection (oral: 3%), flatulence (IV: 1%)
Hematologic & oncologic: Febrile neutropenia (IV: 8%), neutropenia (PO: 4%), petechia (IV: 2%), anemia (IV: 1%), bruise (IV: 1%)
Infection: Fungal infection (6%), herpes virus infection (PO: 6%), serious infection (IV: 6%), viral infection (IV: 6%), herpes zoster infection (2% to 4%)
Neuromuscular & skeletal: Back pain (8%), arthralgia (≤7%), arthritis (PO: ≤7%), asthenia (IV: 6%), myalgia (IV: 6%)
Respiratory: Cough (IV: 7%), bronchitis (PO: 5%), dyspnea (IV: 5%), abnormal breath sounds (IV: 4%), rales (IV: 1%)
Frequency not defined:
Cardiovascular: Edema (IV), phlebitis (IV)
Dermatologic: Cellulitis (IV), erythema of skin (IV), erythematous rash (IV), macular eruption (IV), maculopapular rash (IV), papular rash (IV), pruritic rash (IV), pustular rash (IV)
Hematologic & oncologic: Malignant neoplasm of ovary (PO), purpuric disease (IV)
Infection: Bacteremia (IV), coccidioidomycosis (PO), localized infection (IV), septicemia (IV)
Local: Bleeding at injection site (IV), localized edema (IV)
Renal: Pyelonephritis (PO)
<1%, postmarketing, and/or case reports: Acute renal failure, aplastic anemia, autoimmune hemolytic anemia, basal cell carcinoma, cardiac failure, cervical carcinoma, confusion, conjunctivitis, diplopia, disorientation, eosinophilia, graft versus host disease, hemolytic anemia, hepatic injury, impaired consciousness, increased serum bilirubin, increased serum transaminases, interstitial pulmonary disease, malignant melanoma, mucous membrane ulceration, myelodysplastic syndrome, myocarditis, opportunistic infection, pancreatic adenocarcinoma, pancytopenia, paralysis, paraplegia, peripheral sensory neuropathy, pharyngeal edema, pneumonia, pneumonitis, polyneuropathy, progressive multifocal leukoencephalopathy, pulmonary fibrosis, pulmonary infiltrates, renal failure syndrome, renal insufficiency, respiratory tract infection, seizure, septic shock, severe neurotoxicity, status epilepticus, Stevens-Johnson syndrome, tuberculosis, tumor lysis syndrome, urticaria, vertigo, viral skin infection
Warnings/Precautions
Concerns related to adverse effects:
- Bone marrow suppression: [US Boxed Warning]: Injection: Dose-dependent myelosuppression (neutropenia, anemia, and thrombocytopenia) is common and generally reversible. Lymphopenia has also been reported in patients receiving oral tablets for multiple sclerosis (MS). Use with caution in patients with preexisting hematologic or immunologic abnormalities and patients receiving other drugs that affect the hematologic profile concurrently or prior to treatment with cladribine. Monitor blood counts at baseline and during and after treatment.
- Cardiotoxicity: Cardiotoxicity, including life-threatening acute cardiac failure with myocarditis, has been reported.
- Fever: Treatment is associated with fever (≥100°F), with or without neutropenia, and is observed more commonly in the first month of treatment.
- Graft-versus-host disease: Graft-versus-host disease (GVHD) has been reported rarely in cladribine treated patients following transfusions of nonirradiated blood. Consult with a hematologist; irradiation of cellular blood components prior to transfusion is recommended.
- Hepatotoxicity: Serious liver injury or liver injury leading to treatment discontinuation has been reported within a few weeks to several months after initiation with cladribine. Monitor liver function. If patient develops unexplained liver enzyme elevations or symptoms of liver dysfunction, including abdominal pain, anorexia, dark urine, fatigue, jaundice, or unexplained nausea or vomiting, interrupt or discontinue treatment with cladribine.
- Hypersensitivity: Hypersensitivity reactions have occurred. Discontinue cladribine if a hypersensitivity reaction is suspected.
- Infection: Serious and potentially fatal infections (bacterial, viral, and fungal) have been reported. Due to neutropenia and T-cell depletion, risk versus benefit of treatment should be evaluated in patients with active infections. If active infection is present, manage appropriately prior to administering cladribine (Grever 2017). For the treatment of MS, screen for HIV, tuberculosis, and hepatitis B and C status prior to the first and second treatment course. Consider interrupting or delaying oral cladribine treatment in patients with lymphocyte counts <500/mm3 and signs or symptoms of infections, including herpes infections. If lymphocyte counts <200 cells/mm3, administer anti-herpes prophylaxis. Initiating oral cladribine during concomitant treatment with immunosuppressive or myelosuppressive is not recommended.
- Malignancy: [US Boxed Warning]: Oral tablet: Treatment with cladribine oral tablets may increase the risk of malignancy. After the completion of 2 courses of treatment, do not administer cladribine oral tablets for 2 years. Cladribine oral tablets are contraindicated in patients with current malignancy. In patients with prior malignancy or with increased risk of malignancy, evaluate the benefits and risks of the use of cladribine oral tablets on an individual patient basis. Follow standard cancer screening guidelines in patients treated with cladribine oral tablets.
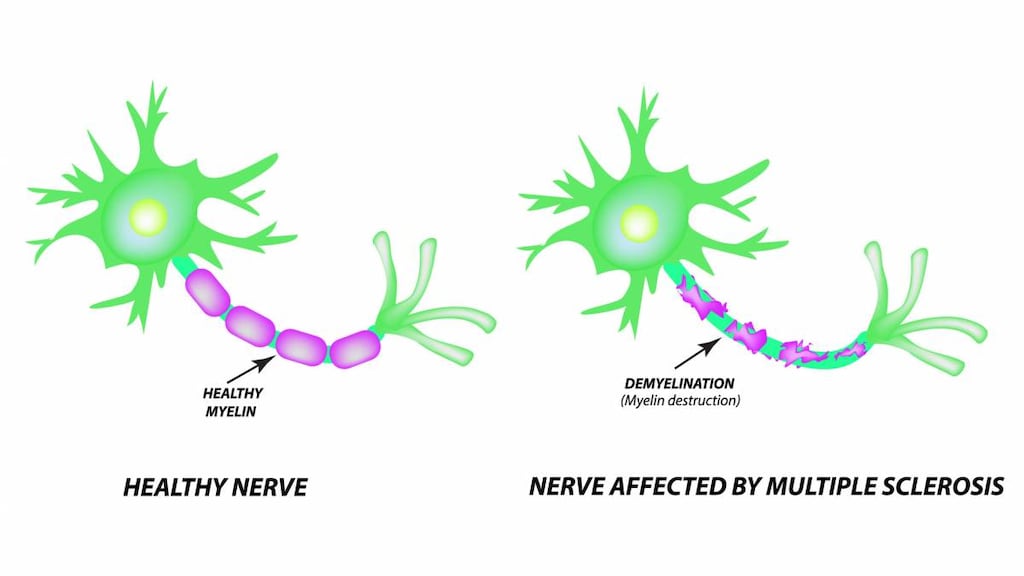
- Neurotoxicity: [US Boxed Warning]: Injection: Serious, dose-related neurologic toxicity (including irreversible paraparesis and quadriparesis) has been reported with continuous infusions of higher doses (4 to 9 times the approved dose); may also occur at approved doses (rare). Neurotoxicity may be delayed and may present as progressive, irreversible motor weakness of the upper and/or lower extremities; diagnostics with electromyography and nerve conduction studies were consistent with demyelinating disease. Neurotoxicity after high-dose administration was first noted 35 to 84 days after therapy initiation.
- Progressive multifocal leukoencephalopathy: Cases of progressive multifocal leukoencephalopathy (PML) due to the John Cunningham (JC) virus have been reported in patients receiving parenteral cladribine for oncologic indications. Symptoms progress over days to weeks and may include progressive weakness on one side of the body or clumsiness of limbs, vision disturbances, and mental status changes. Obtain baseline MRI before treatment initiation. At the first sign or symptom suggestive of PML, perform a diagnostic evaluation and withhold therapy. MRI findings may be apparent before patients are symptomatic. Monitoring with brain MRI for signs that may be consistent with PML may be beneficial and allow for an early diagnosis of PML.
- Renal toxicity: [US Boxed Warning]: Injection: Acute nephrotoxicity (eg, acidosis, anuria, increased serum creatinine), possibly requiring dialysis, has been reported with high doses (4 to 9 times the approved dose), particularly when administered with other nephrotoxic agents. Per the manufacturer, nephrotoxicity has not occurred when used at the dose approved for hairy cell leukemia.
- Tumor lysis syndrome: With high tumor burden, tumor lysis syndrome and subsequent hyperuricemia may occur (rare); consider antihyperuricemics and hydrate accordingly.
Disease-related concerns:
- Hepatic impairment: Use with caution in patients with hepatic impairment.
- Renal impairment: Use with caution in patients with renal impairment.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pregnancy: [US Boxed Warning]: Oral tablet:Cladribine oral tablets are contraindicated for use in pregnant women and in women and men of reproductive potential who do not plan to use effective contraception because of the potential for fetal harm. Malformations and embryolethality occurred in animals. Exclude pregnancy before the start of treatment with cladribine oral tablets in females of reproductive potential. Advise females and males of reproductive potential to use effective contraception during cladribine oral tablet dosing and for 6 months after the last dose in each treatment course. Stop cladribine oral tablets if the patient becomes pregnant.
Dosage form specific issues:
- Benzyl alcohol and derivatives: Injection: Weekly (7-day) infusion preparation recommends further dilution with bacteriostatic normal saline which contains benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol with caution in neonates. See manufacturer's labeling.
Other warnings/precautions:
- Experienced physician: [US Boxed Warning]: Injection: Should be administered under the supervision of an experienced cancer chemotherapy physician.
- Vaccines: Administration of live-attenuated or live vaccines is not recommended during treatment with cladribine (may increase the risk of infection due to immunosuppression). When using for the treatment of MS, complete necessary immunizations at least 4 to 6 weeks prior to initiating cladribine. Determine if patient has a history varicella or vaccination for VZV; if not, test for VZV antibodies and consider vaccinations for antibody-negative patients.
Monitoring Parameters
IV: CBC with differential (particularly during the first 4 to 8 weeks post-treatment), renal and hepatic function; bone marrow biopsy (after CBC has normalized, to confirm treatment response); monitor for fever; monitor for signs/symptoms of neurotoxicity and infection
Oral tablet: CBC including lymphocyte count (before each treatment course, 2 and 6 months after the start of each yearly course [if 2-month lymphocyte <200 cells/mm3, monitor monthly until month 6], and periodically during and after treatment); evaluate HIV, tuberculosis, hepatitis B (HBV) and hepatitis C (HCV) status (prior to each treatment course); evaluate varicella zoster virus VZV antibody status (prior to treatment initiation); pregnancy test (prior to treatment in females of reproductive potential); liver function tests (serum aminotransferase, alkaline phosphatase and total bilirubin levels) (prior to each treatment course and as clinically appropriate); MRI (at baseline [within 3 months] prior to first treatment course); signs or symptoms of progressive multifocal leukoencephalopathy (if PML is suspected, obtain brain MRI scan); standard cancer screening; signs or symptoms of acute infection
Pregnancy
Pregnancy Considerations
Based on the mechanism of action and data from animal reproduction studies, in utero exposure to cladribine is expected to cause fetal harm. Females of reproductive potential should use highly effective contraception during therapy regardless of the route of administration/indication for treatment.
[US Boxed Warning]: Oral tablet: Cladribine is contraindicated for use in pregnant women and in women and men of reproductive potential who do not plan to use effective contraception because of the potential for fetal harm. Malformations and embryolethality occurred in animals. Exclude pregnancy before the start of treatment with cladribine in females of reproductive potential. Advise females and males of reproductive potential to use effective contraception during cladribine dosing and for 6 months after the last dose in each treatment course. Stop cladribine if the patient becomes pregnant. Pregnancy should be excluded in females of reproductive potential prior to each course of cladribine. The effect of cladribine on hormonal contraceptives is not known, use of a barrier method is recommended in addition to systemic hormonal contraceptives during therapy and for 4 weeks after the last cladribine dose.
In general, disease-modifying therapies for multiple sclerosis are stopped prior to a planned pregnancy, and not initiated during pregnancy, except in females at high risk of multiple sclerosis activity (AAN [Rae-Grant 2018]). Consider use of agents other than cladribine for females at high risk of disease reactivation who are planning a pregnancy. Delaying pregnancy is recommended for females with persistent high disease activity; when disease-modifying therapy is needed in these patients, other agents are preferred (ECTRIMS/EAN [Montalban 2018]).
Information related to the use of cladribine for the treatment of hairy cell leukemia in pregnancy is limited (Daver 2013).
A pregnancy registry is available for all cancers diagnosed during pregnancy at Cooper Health (877-635-4499).
Patient Education
What is this drug used for?
Tablets:
- It is used to treat MS (multiple sclerosis).
Injection:
- It is used to treat a type of leukemia.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Nausea
- Vomiting
- Diarrhea
- Injection site irritation
- Muscle pain
- Common cold symptoms
- Back pain
- Joint pain
- Trouble sleeping
- Lack of appetite
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Infection
- Kidney problems like unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain
- Burning, numbness, tingling, or weakness in arms or legs
- Abnormal heartbeat
- Bleeding like vomiting blood or vomit that looks like coffee grounds; coughing up blood; blood in the urine; black, red, or tarry stools; bleeding from the gums; abnormal vaginal bleeding; bruises without a reason or that get bigger; or any severe or persistent bleeding
- Shortness of breath
- Excessive weight gain
- Swelling of arms or legs
- Fast heartbeat
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin
- Severe headache
- Severe dizziness
- Passing out
- Vision changes
- Depression
- Progressive multifocal leukoencephalopathy like confusion, depression, trouble with memory, behavioral changes, change in strength on one side is greater than the other, trouble speaking, change in balance, or vision changes
- Severe loss of strength and energy
- Tumor lysis syndrome like fast heartbeat or abnormal heartbeat; any passing out; unable to pass urine; muscle weakness or cramps; nausea, vomiting, diarrhea or lack of appetite; or feeling sluggish
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.