Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
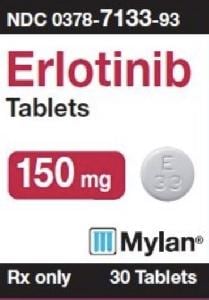
Tablet, Oral:
Tarceva: 25 mg [contains fd&c yellow #6 (sunset yellow)]
Tarceva: 100 mg, 150 mg
Generic: 25 mg, 100 mg, 150 mg
Pharmacology
Mechanism of Action
Reversibly inhibits overall epidermal growth factor receptor (HER1/EGFR) - tyrosine kinase activity. Intracellular phosphorylation is inhibited which prevents further downstream signaling, resulting in cell death. Erlotinib has higher binding affinity for EGFR exon 19 deletion or exon 21 L858R mutations than for the wild type receptor.
Pharmacokinetics/Pharmacodynamics
Absorption
Oral: ~60% on an empty stomach; food increases to ~100%
Distribution
232 L
Metabolism
Hepatic, via CYP3A4 (major), CYP1A1 (minor), CYP1A2 (minor), and CYP1C (minor)
Excretion
Primarily as metabolites: Feces (83%; 1% as unchanged drug); urine (8%; <1% as unchanged drug)
Time to Peak
Plasma: 4 hours
Half-Life Elimination
36.2 hours
Protein Binding
93% to albumin and alpha1-acid glycoprotein
Use in Specific Populations
Special Populations Note
Cigarette smoking: Smoking reduces erlotinib AUC by 64% (compared to former/never smokers); smokers had a 24% higher rate of erlotinib clearance.
Use: Labeled Indications
Non-small cell lung cancer, metastatic: Treatment of metastatic non-small cell lung cancer (NSCLC) in tumors with epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations as detected by an approved test either as first-line, maintenance, or as second or greater line treatment after progression following at least 1 prior chemotherapy regimen.
Limitations of use: Use in combination with platinum-based chemotherapy is not recommended. Safety and efficacy of treatment for metastatic NSCLC with EGFR mutations other than exon 19 deletion or exon 21 (L858R) substitution have not been established.
Pancreatic cancer: First-line treatment of locally advanced, unresectable, or metastatic pancreatic cancer (in combination with gemcitabine)
Contraindications
There are no contraindications listed in the manufacturer’s US labeling.
Canadian labeling: Hypersensitivity to erlotinib or any component of the formulation
Dosage and Administration
Dosing: Adult
Non-small cell lung cancer (NSCLC), metastatic, in patients with EGFR exon 19 deletions or exon 21 (L858R) substitution mutations: Oral: 150 mg once daily until disease progression or unacceptable toxicity (Capuzzo 2010; Rosell 2012; Shepherd 2005).
Pancreatic cancer: Oral: 100 mg once daily (in combination with gemcitabine); continue until disease progression or unacceptable toxicity (Moore 2007).
Dosage adjustment for concomitant CYP inhibitors/inducers:
CYP3A4 inhibitors (strong): Avoid concurrent use if possible; reduce erlotinib dose for severe adverse reactions if erlotinib is administered concomitantly with strong CYP3A4 inhibitors. Dose reduction should be done in decrements of 50 mg (after toxicity has resolved to baseline or ≤ grade 1).
Concomitant CYP3A4 and CYP1A2 inhibitor (eg, ciprofloxacin): Avoid concurrent use if possible; if concomitant use cannot be avoided, reduce dose in decrements of 50 mg if severe adverse reactions occur (after toxicity has resolved to baseline or ≤ grade 1).
CYP3A4 inducers: Avoid concurrent use if possible; if concomitant administration with CYP3A4 inducers cannot be avoided, increase erlotinib dose in 50 mg increments at 2-week intervals to a maximum of 450 mg; reduce erlotinib dose to recommended starting dose when CYP3A4 inducer is discontinued.
CYP1A2 inducers: Avoid concurrent moderate CYP1A2 inducers if possible. If unavoidable, increase dose at 2-week intervals in 50 mg increments to a maximum dose of 300 mg (with careful monitoring); immediately reduce erlotinib dose to recommended starting dose (based on indication) upon discontinuation of the moderate CYP1A2 inducer.
Dosage adjustment for concomitant smoking: Avoid tobacco smoking if possible. If unavoidable, increase dose at 2-week intervals in 50 mg increments to a maximum dose of 300 mg (with careful monitoring); immediately reduce erlotinib dose to recommended starting dose (based on indication) upon smoking cessation.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Adjustment for Toxicity
Dermatologic toxicity:
Bullous, blistering, or exfoliative skin toxicity (severe): Discontinue treatment.
Severe rash (unresponsive to medical management): Withhold treatment; may reinitiate with a 50 mg dose reduction after toxicity has resolved to baseline or ≤ grade 1.
Gastrointestinal toxicity:
Diarrhea: Manage with loperamide; in persistent, severe diarrhea (unresponsive to loperamide) or dehydration due to diarrhea, withhold treatment; may reinitiate with a 50 mg dose reduction after toxicity has resolved to baseline or ≤ grade 1.
Gastrointestinal perforation: Discontinue treatment.
Ocular toxicities:
Acute or worsening ocular toxicities (eg, eye pain): Interrupt and consider discontinuing treatment. If therapy is resumed, reinitiate with a 50 mg dose reduction after toxicity has resolved to baseline or ≤ grade 1.
Corneal perforation or severe ulceration: Discontinue treatment.
Keratitis (grade 3 or 4 or grade 2 persisting >2 weeks): Withhold treatment; may reinitiate with a 50 mg dose reduction after toxicity has resolved to baseline or ≤ grade 1.
Pulmonary symptoms: Acute onset (or worsening) of pulmonary symptoms (eg, dyspnea, cough, fever): Withhold treatment while evaluating for drug-induced interstitial lung disease; if resuming treatment, reinitiate with a 50 mg dose reduction after symptoms resolve to grade 1 or lower. Discontinue permanently with development of interstitial lung disease
Extemporaneously Prepared
A 10 mg/mL oral suspension may be prepared using erlotinib 150 mg tablets and Ora-Plus:Ora-Sweet (1:1 vehicle). Determine necessary quantity of erlotinib 150 mg tablets; crush the tablets in a glass mortar and triturate to a fine powder (estimated powder volume for each erlotinib 150 mg tablet is 0.25 mL). Measure the necessary volume of Ora-Plus and add to the powder by geometric dilution until a smooth suspension is created. Measure the necessary volume of Ora-Sweet and add to the suspension. Transfer to an amber plastic bottle and label “Shake Well Before Use”, “Do Not Refrigerate” and “Use by (date)”. Suspension is stable for at least 28 days at room temperature (do not refrigerate due to potential increased viscosity).
Li Q, Liu Z, Kolli S, et al. Stability of extemporaneous erlotinib, lapatinib, and imatinib oral suspension. Am J Health Syst Pharm. 2016;73(17):1331-1337.27543577
Administration
The manufacturer recommends administration on an empty stomach (at least 1 hour before or 2 hours after the ingestion of food). Avoid concomitant use with proton pump inhibitors (if possible). If taken with an H2-receptor antagonist (eg, ranitidine), administer erlotinib 10 hours after the H2-receptor antagonist dose and at least 2 hours prior to the next H2- receptor dose. If an antacid is necessary, separate dosing by several hours.
For patients unable to swallow whole, tablets may be dissolved in 100 mL water and administered orally or via feeding tube (silicone-based); to ensure full dose is received, rinse container with 40 mL water, administer residue and repeat rinse; administer immediately after preparation (data on file, Genentech [contact product manufacturer to obtain current information]; Siu 2007; Soulieres 2004). If necessary, an oral suspension may be prepared (see Extemporaneously Prepared).
Dietary Considerations
Avoid grapefruit and grapefruit juice.
Storage
Store at 25°C (77°F); excursions are permitted between 15°C to 30°C (59°F to 86°F).
An oral suspension (10 mg/mL) prepared using erlotinib 150 mg tablets and Ora-Plus:Ora-Sweet (1:1 vehicle) is stable for at least 28 days at room temperature; do not refrigerate due to potential increased viscosity (Li 2016).
Erlotinib Images
Drug Interactions
Antacids: May decrease the serum concentration of Erlotinib. Management: Separate the administration of erlotinib and any antacid by several hours in order to minimize the risk of a significant interaction. Consider therapy modification
Aprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Bosentan: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Ciprofloxacin (Systemic): May increase the serum concentration of Erlotinib. Management: Avoid use of this combination when possible. When the combination must be used, monitor the patient closely for the development of severe adverse reactions, and if such severe reactions occur, reduce the erlotinib dose (in 50 mg decrements). Consider therapy modification
Clofazimine: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Conivaptan: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May decrease the serum concentration of Erlotinib. Management: Avoid combination if possible. If combination must be used, increase erlotinib dose by 50 mg increments every 2 weeks as tolerated, to a maximum of 450 mg/day. Consider therapy modification
CYP3A4 Inhibitors (Moderate): May decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CYP3A4 Inhibitors (Strong): May increase the serum concentration of Erlotinib. Management: Avoid use of this combination when possible. When the combination must be used, monitor the patient closely for the development of severe adverse reactions, and if such severe reactions occur, reduce the erlotinib dose (in 50 mg decrements). Consider therapy modification
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Duvelisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Enzalutamide: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Concurrent use of enzalutamide with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP3A4 substrate should be performed with caution and close monitoring. Consider therapy modification
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Erdafitinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
FluvoxaMINE: May increase the serum concentration of Erlotinib. Management: Avoid use of this combination when possible. When the combination must be used, monitor the patient closely for the development of severe adverse reactions, and if such severe reactions occur, reduce the erlotinib dose (in 50 mg decrements). Consider therapy modification
Fosaprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fosnetupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fosphenytoin-Phenytoin: Erlotinib may increase the serum concentration of Fosphenytoin-Phenytoin. Fosphenytoin-Phenytoin may decrease the serum concentration of Erlotinib. Management: Avoid use of erlotinib with phenytoin when possible. If required, increase erlotinib dose by 50 mg increments at 2 week intervals, as tolerated, to a max of 450 mg/day. Avoid combination
Fusidic Acid (Systemic): May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Grapefruit Juice: May increase the serum concentration of Erlotinib. Management: Avoid use of this combination when possible. When the combination must be used, monitor the patient closely for the development of severe adverse reactions, and if such severe reactions occur, reduce the erlotinib dose (in 50 mg decrements). Consider therapy modification
Histamine H2 Receptor Antagonists: May decrease the serum concentration of Erlotinib. Management: Avoid H2-antagonists in patients receiving erlotinib when possible. If concomitant treatment cannot be avoided, erlotinib should be dosed once daily, 10 hours after and at least 2 hours before H2-antagonist dosing. Consider therapy modification
Idelalisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Ivosidenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Larotrectinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Leflunomide: May decrease the serum concentration of Erlotinib. Management: Avoid the concomitant use of erlotinib and leflunomide if possible. If concomitant use is unavoidable, increase the erlotinib dose by 50 mg increments at 2-week intervals to a maximum of 300 mg. Consider therapy modification
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
MiFEPRIStone: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Minimize doses of CYP3A4 substrates, and monitor for increased concentrations/toxicity, during and 2 weeks following treatment with mifepristone. Avoid cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, and tacrolimus. Consider therapy modification
Mitotane: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Doses of CYP3A4 substrates may need to be adjusted substantially when used in patients being treated with mitotane. Consider therapy modification
Netupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Palbociclib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Proton Pump Inhibitors: May decrease the serum concentration of Erlotinib. Avoid combination
Rifabutin: May decrease the serum concentration of Erlotinib. Management: Avoid combination if possible. If combination must be used, increase erlotinib dose by 50 mg increments every 2 weeks as tolerated, to a maximum of 450 mg/day. Consider therapy modification
Rifapentine: May decrease the serum concentration of Erlotinib. Management: Avoid combination if possible. If combination must be used, increase erlotinib dose by 50 mg increments every 2 weeks as tolerated, to a maximum of 450 mg/day. Consider therapy modification
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Simeprevir: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
St John's Wort: May decrease the serum concentration of Erlotinib. Management: Avoid combination if possible. If combination must be used, increase erlotinib dose by 50 mg increments every 2 weeks as tolerated, to a maximum of 450 mg/day. Consider therapy modification
Stiripentol: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Use of stiripentol with CYP3A4 substrates that are considered to have a narrow therapeutic index should be avoided due to the increased risk for adverse effects and toxicity. Any CYP3A4 substrate used with stiripentol requires closer monitoring. Consider therapy modification
Teriflunomide: May decrease the serum concentration of Erlotinib. Management: Avoid the concomitant use of erlotinib and teriflunomide if possible. If concomitant use is unavoidable, increase the erlotinib dose by 50 mg increments at 2-week intervals to a maximum of 300 mg. Consider therapy modification
Tobacco (Smoked): May decrease the serum concentration of Erlotinib. Management: Avoid cigarette smoking during treatment with erlotinib whenever possible. If combined, increase erlotinib dose by 50 mg increments at 2-week intervals to a maximum of 300 mg daily. Consider therapy modification
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Warfarin: Erlotinib may increase the serum concentration of Warfarin. Monitor therapy
Adverse Reactions
Adverse reactions reported with monotherapy:
>10%:
Cardiovascular: Chest pain (≤18%)
Central nervous system: Fatigue (9% to 52%)
Dermatologic: Skin rash (49% to 85%; grade 3: 5% to 13%; grade 4: <1%; median onset: 8 days), xeroderma (4% to 21%), pruritus (7% to 16%), paronychia (4% to 16%), alopecia (14% to 15%), acne vulgaris (6% to 12%)
Gastrointestinal: Diarrhea (20% to 62%; grade 3: 2% to 6%; grade 4: <1%; median onset: 12 days), anorexia (9% to 52%), nausea (23% to 33%), decreased appetite (≤28%), vomiting (13% to 23%), mucositis (≤18%), stomatitis (11% to 17%), abdominal pain (3% to 11%), constipation (≤8%)
Genitourinary: Urinary tract infection (≤4%)
Hematologic & oncologic: Anemia (≤11%; grade 4: 1%)
Infection: Increased susceptibility to infection (4% to 24%)
Neuromuscular & skeletal: Weakness (≤53%), back pain (19%), arthralgia (≤13%), musculoskeletal pain (11%)
Ophthalmic: Conjunctivitis (12% to 18%), keratoconjunctivitis sicca (12%)
Respiratory: Cough (33% to 48%), dyspnea (41% to 45%; grades 3/4: 8% to 28%)
Miscellaneous: Fever (≤11%)
1% to 10%:
Cardiovascular: Peripheral edema (≤5%)
Central nervous system: Pain (≤9%), headache (≤7%), anxiety (≤5%), dizziness (≤4%), insomnia (≤4%), neurotoxicity (≤4%), paresthesia (≤4%), voice disorder (≤4%)
Dermatologic: Folliculitis (≤8%), nail disease (≤7%), exfoliative dermatitis (5%), hypertrichosis (5%), skin fissure (5%), acneiform eruption (4% to 5%), erythema (≤5%), dermatitis (4%), erythematous rash (≤4%), palmar-plantar erythrodysesthesia (≤4%), bullous dermatitis
Endocrine & metabolic: Weight loss (4% to 5%)
Gastrointestinal: Dyspepsia (≤5%), xerostomia (≤3%), taste disorder (≤1%)
Hematologic & oncologic: Lymphocytopenia (≤4%; grade 3: 1%), leukopenia (≤3%), thrombocytopenia (≤1%)
Hepatic: Hyperbilirubinemia (7%; grade 3: ≤1%), increased serum ALT (grade 2: 2% to 4%; grade 3: 1% to 3%), increased gamma-glutamyl transferase (≤4%), hepatic failure (≤1%)
Neuromuscular & skeletal: Muscle spasm (≤4%), musculoskeletal chest pain (≤4%), ostealgia (≤4%)
Otic: Tinnitus (≤1%)
Renal: Increased serum creatinine (≤1%), renal failure (≤1%),
Respiratory: Nasopharyngitis (≤7%), epistaxis (≤4%), pulmonary embolism (≤4%), respiratory tract infection (≤4%), pneumonitis (3%), pulmonary fibrosis (3%)
<1%: Interstitial pulmonary disease
Adverse reactions reported with combination (erlotinib plus gemcitabine) therapy:
>10%:
Cardiovascular: Edema (37%), thrombosis (grades 3/4: 11%)
Central nervous system: Fatigue (73% to 79%), depression (19%), dizziness (15%), headache (15%), anxiety (13%)
Dermatologic: Skin rash (70%), alopecia (14%)
Gastrointestinal: Nausea (60%), anorexia (52%), diarrhea (48%), abdominal pain (46%), vomiting (42%), weight loss (39%), stomatitis (22%), dyspepsia (17%), flatulence (13%)
Hepatic: Increased serum ALT (grade 2: 31%, grade 3: 13%, grade 4: <1%), increased serum AST (grade 2: 24%, grade 3: 10%, grade 4 <1%), hyperbilirubinemia (grade 2: 17%, grade 3: 10%, grade 4: <1%)
Infection: Increased susceptibility to infection (39%)
Neuromuscular & skeletal: Ostealgia (25%), myalgia (21%), neuropathy (13%), rigors (12%)
Respiratory: Dyspnea (24%), cough (16%)
Miscellaneous: Fever (36%)
1% to 10%:
Cardiovascular: Cardiac arrhythmia (<5%), syncope (<5%), deep vein thrombosis (4%), cerebrovascular accident (3%; including cerebral hemorrhage), myocardial infarction (2%)
Gastrointestinal: Intestinal obstruction (<5%), pancreatitis (<5%)
Hematologic & oncologic: Hemolytic anemia (<5%), microangiopathic hemolytic anemia with thrombocytopenia (1%)
Renal: Renal insufficiency (<5%), renal failure (1%)
Respiratory: Interstitial pulmonary disease (<3%)
<1%: Bullous dermatitis, exfoliative dermatitis, hepatic failure
Mono- or combination therapy: <1%, postmarketing, and/or case reports: Acute peptic ulcer with hemorrhage, bronchiolitis, corneal perforation, corneal ulcer, decreased lacrimation, episcleritis, gastritis, gastrointestinal hemorrhage, gastrointestinal perforation, hearing loss, hematemesis, hematochezia, hepatorenal syndrome, hepatotoxicity, hirsutism, hyperpigmentation, hypokalemia, increased eyelash thickness, increased growth in number of eyelashes, keratitis, melena, misdirected growth of eyelashes, myopathy (in combination with statin therapy), ocular inflammation, peptic ulcer, rhabdomyolysis (in combination with statin therapy), skin photosensitivity, skin rash (acneiform; sparing prior radiation field), Stevens-Johnson syndrome, toxic epidermal necrolysis, tympanic membrane perforation, uveitis
Warnings/Precautions
Concerns related to adverse effects:
- Cardiovascular events: Cerebrovascular accidents, MI, and myocardial ischemia have been reported (some fatal).
- Dermatologic toxicity: Bullous, blistering, and/or exfoliating skin conditions, some suggestive of Stevens-Johnson or toxic epidermal necrolysis (TEN), have been reported (some fatal). An acne-like rash commonly appears on the face, back, and upper chest. Generalized or severe acneiform, erythematous or maculopapular rash may occur. Skin rash may correlate with treatment response and prolonged survival (Saif 2008); management of skin rashes that are not serious should include alcohol-free lotions, topical antibiotics, or topical corticosteroids, or if necessary, oral antibiotics and systemic corticosteroids; avoid sunlight. Reduce dose or temporarily interrupt treatment for severe skin reactions; discontinue treatment for bullous, blistering, or exfoliative skin toxicity.
- Gastrointestinal (GI) perforation: GI perforation (including fatalities) has been reported; risk for perforation is increased with concurrent anti-angiogenic agents, corticosteroids, NSAIDs, and/or taxane based-chemotherapy, and patients with history of peptic ulcers or diverticular disease. Permanently discontinue in patients who develop perforation.
- Hematologic effects: Microangiopathic hemolytic anemia (MAHA) with thrombocytopenia has been reported (rarely) with erlotinib in combination with gemcitabine.
- Hemorrhage: Elevated INR and bleeding events (including fatal hemorrhage) have been reported when erlotinib was administered concomitantly with warfarin; monitor prothrombin time and INR closely.
- Hepatotoxicity: Hepatic failure and hepatorenal syndrome have been reported (some fatal), particularly in patients with baseline hepatic impairment (although have also been observed in patients with normal hepatic function). Monitor liver function (transaminases, bilirubin, and alkaline phosphatase); patients with any hepatic impairment (total bilirubin >ULN; Child-Pugh class A, B, or C) should be closely and more frequently monitored, including those with hepatic disease due to tumor burden. Increased monitoring of liver function is required in patients with preexisting hepatic impairment or biliary obstruction. Dosage reduction, interruption, or discontinuation may be necessary for changes in hepatic function. Use with extreme caution in patients with total bilirubin >3 times ULN. Interrupt therapy if total bilirubin is >3 times ULN or transaminases are >5 times ULN in patients without preexisting hepatic impairment. In patients with baseline hepatic dysfunction or biliary obstruction, interrupt therapy if bilirubin doubles or transaminases triple from baseline values.
- Ocular toxicity: Corneal perforation and ulceration have been reported; decreased tear production, abnormal eyelash growth, keratoconjunctivitis sicca, or keratitis have also been reported and are known risk factors for corneal ulceration/perforation. Interrupt or discontinue treatment in patients presenting with eye pain or other acute or worsening ocular symptoms. Consider a baseline ophthalmologic exam and reassess for ocular toxicities at 4 to 8 weeks after treatment initiation (Renouf 2012).
- Pulmonary toxicity: Rare, sometimes fatal, interstitial lung disease (ILD) has occurred; symptoms include acute respiratory distress syndrome, interstitial pneumonia, obliterative bronchiolitis, pneumonitis (including radiation and hypersensitivity), pulmonary fibrosis, and pulmonary infiltrates. The onset of symptoms has been within 5 days to more than 9 months after treatment initiation (median: 39 days). Interrupt treatment for unexplained new or worsening pulmonary symptoms (dyspnea, cough, and fever); permanently discontinue for confirmed ILD.
- Renal impairment: Acute renal failure (some fatal), renal insufficiency, and hepatorenal syndrome have been reported, either secondary to hepatic impairment at baseline or due to severe dehydration; use with caution in patients with or at risk for renal impairment. Monitor closely for dehydration; monitor renal function and electrolytes in patients at risk for dehydration. If severe renal impairment develops, interrupt therapy until toxicity resolves.
Disease-related concerns:
- NSCLC: Some factors which correlate positively with response to EGFR-tyrosine kinase inhibitor (TKI) therapy in NSCLC include patients who have never smoked, EGFR mutation, and patients of Asian origin. EGFR mutations, specifically exon 19 deletions and exon 21 mutation (L858R), are associated with better response to erlotinib in patients with NSCLC (Riely 2006). Erlotinib treatment is not recommended in patients with NSCLC with K-ras mutations; they are not likely to benefit from erlotinib treatment (Eberhard 2005; Miller 2008). K-ras mutations correlated with poorer outcome with EGFR-TKI therapy in patients with NSCLC (Jackman 2009; Masarelli 2007; Shepherd 2005). The cobas EGFR mutation test has been approved to detect EGFR mutation for NSCLC treatment.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
- Drugs affecting gastric pH: Avoid concomitant use with proton pump inhibitors. If taken with an H2-receptor antagonist (eg, ranitidine), administer erlotinib 10 hours after the H2-receptor antagonist dose and at least 2 hours prior to the next H2-receptor dose. If an antacid is necessary, separate dosing by several hours.
Special populations:
- Smokers: Erlotinib levels may be lower in patients who smoke; advise patients to stop smoking. Smokers treated with 300 mg/day exhibited steady-state erlotinib levels comparable to former- and never-smokers receiving 150 mg/day (Hughes 2009).
Other warnings/precautions:
- Appropriate use: Concurrent erlotinib plus platinum-based chemotherapy is not recommended for treatment of locally-advanced or metastatic NSCLC due to a lack of clinical benefit. Treatment in patients with metastatic NSCLC with EGFR mutations other than exon 19 deletion or exon 21 (L858R) substitution has not been evaluated. Select patients for metastatic NSCLC treatment based on EGFR exon 19 deletions and exon 21 mutation (L858R) in tumor or plasma specimens; if these mutations are not detected in plasma specimen, tumor tissue (if available) may be tested.
- Lactose intolerance: Product may contain lactose; avoid use in patients with Lapp lactase deficiency, glucose-galactose malabsorption, or glucose intolerance.
Monitoring Parameters
Liver function tests (transaminases, bilirubin, and alkaline phosphatase [periodic], monitor more frequently with worsening liver function); renal function tests (periodic) and serum electrolytes (in patients at risk for dehydration; periodic); prothrombin time and INR (in patients on concomitant warfarin therapy); EGFR mutation status in patients with NSCLC adenocarcinoma (Keedy 2011); the cobas EGFR mutation test has been approved to detect EGFR mutation for first-line NSCLC treatment, smoking status.
Consider a baseline ophthalmologic exam and reassess for ocular toxicities at 4 to 8 weeks after treatment initiation (Renouf 2012). Monitor hydration status; monitor for signs/symptoms of pulmonary toxicity, dermatologic toxicity, and ocular toxicity.
Pregnancy
Pregnancy Considerations
Erlotinib crosses the placenta (Ji 2015; Jovelet 2015). Information related to the use of erlotinib in pregnancy is limited (Ji 2015; Rivas 2012; Zambelli 2008). Based on the mechanism of action and data from animal reproduction studies, erlotinib may cause fetal harm if administered in pregnancy. Females of reproductive potential should use effective contraception during treatment and for at least 1 month after the last erlotinib dose.
Patient Education
What is this drug used for?
- It is used to treat lung cancer and pancreatic cancer.
Frequently reported side effects of this drug
- Itching
- Nausea
- Diarrhea
- Lack of appetite
- Mouth sores
- Mouth irritation
- Headache
- Back pain
- Joint pain
- Muscle pain
- Hair or nail changes
- Dry skin
- Weight loss
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Infection
- Acne
- Skin irritation
- Redness or irritation of palms of hands or soles of feet
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Kidney problems like unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain
- Severe pulmonary disorder like lung or breathing problems like difficulty breathing, shortness of breath, or a cough that is new or worse
- Severe cerebrovascular disease like change in strength on one side is greater than the other, difficulty speaking or thinking, change in balance, or vision changes
- Chest pain
- Bruising
- Bleeding
- Severe loss of strength and energy
- Vision changes
- Eye pain
- Severe eye irritation
- Sensitivity to bright lights
- Depression
- Severe abdominal pain
- Vomiting blood
- Black, tarry, or bloody stools
- Stevens-Johnson syndrome/toxic epidermal necrolysis like red, swollen, blistered, or peeling skin (with or without fever); red or irritated eyes; or sores in mouth, throat, nose, or eyes
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.