Boxed Warning
Immunosuppression (Zortress):
Increased susceptibility to infection and the possible development of malignancies, such as lymphoma and skin cancer, may result from immunosuppression.
Only health care providers experienced in immunosuppressive therapy and management of transplant patients should use everolimus. Manage patients receiving the drug in facilities equipped and staffed with adequate laboratory and supportive medical resources. The health care provider responsible for maintenance therapy should have complete information requisite for the follow-up.
Renal graft thrombosis (Zortress):
An increased risk of kidney arterial and venous thrombosis, resulting in graft loss, was reported, mostly within the first 30 days post-transplantation.
Nephrotoxicity (Zortress):
Increased nephrotoxicity can occur with use of standard doses of cyclosporine in combination with everolimus. Therefore, use reduced doses of cyclosporine in combination with everolimus in order to reduce renal dysfunction. It is important to monitor the cyclosporine and everolimus whole blood trough concentrations.
Mortality in heart transplant (Zortress):
Increased mortality, often associated with serious infection, within the first 3 months of post-transplantation was observed in a clinical trial of de novo heart transplant patients receiving immunosuppressive regimens with or without induction therapy. Use in heart transplantation is not recommended.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Afinitor: 2.5 mg, 5 mg, 7.5 mg, 10 mg
Zortress: 0.25 mg, 0.5 mg, 0.75 mg, 1 mg
Generic: 2.5 mg, 5 mg, 7.5 mg
Tablet Soluble, Oral:
Afinitor Disperz: 2 mg, 3 mg, 5 mg
Pharmacology
Mechanism of Action
Everolimus is a macrolide immunosuppressant and a mechanistic target of rapamycin (mTOR) inhibitor which has antiproliferative and antiangiogenic properties, and also reduces lipoma volume in patients with angiomyolipoma. Reduces protein synthesis and cell proliferation by binding to the FK binding protein-12 (FKBP-12), an intracellular protein, to form a complex that inhibits activation of mTOR (mechanistic target of rapamycin) serine-threonine kinase activity. Also reduces angiogenesis by inhibiting vascular endothelial growth factor (VEGF) and hypoxia-inducible factor (HIF-1) expression. Angiomyolipomas may occur due to unregulated mTOR activity in TSC-associated renal angiomyolipoma (Budde 2012); everolimus reduces lipoma volume (Bissler 2013).
Pharmacokinetics/Pharmacodynamics
Absorption
Rapid (Kirchner 2004)
Distribution
Apparent Vd: 128 to 589 L (Zortress); volume of distribution in pediatric renal transplant patients (3 to 16 years) lower than adults (Van Damme-Lombaerts 2002)
Metabolism
Extensively metabolized in the liver via CYP3A4; forms 6 weak metabolites (Afinitor and Zortress)
Excretion
Feces (80%, based on solid organ transplant studies); Urine (~5%, based on solid organ transplant studies); clearance in pediatric renal transplant patients lower than adults possibly due to distributive differences (Van Damme-Lombaerts 2002)
Time to Peak
1 to 2 hours (Afinitor and Zortress)
Half-Life Elimination
~30 hours (Afinitor and Zortress); in pediatric renal transplant patients (3 to 16 years), half-life similar to adult data (Van Damme-Lombaerts 2002)
Protein Binding
~74% (Afinitor and Zortress)
Use in Specific Populations
Special Populations: Hepatic Function Impairment
Afinitor: In one study, subjects with mild, moderate, and severe impairment had a 1.8-fold, 3.2-fold, and 3.6-fold higher AUC, respectively, compared to patients with normal function. In another study, the average AUC in subjects with moderate impairment was twice that of subjects with normal function.
Zortress: The average AUC in subjects with mild impairment was 1.6-fold higher than that of patients with normal hepatic function (following a 10 mg dose); in moderate impairment the AUC was 2.1- to 3.3-fold higher than that of patients with normal hepatic function (following a 2 mg or 10 mg dose); in severe impairment the AUC was 3.6-fold higher than that of patients with normal hepatic function (following a 10 mg dose).
Special Populations: Children
In patients with tuberous sclerosis complex (TSC)-associated subependymal giant cell astrocytoma (SEGA) or TSC-associated partial-onset seizures, mean serum Cmin values when normalized to mg/m2 dose in pediatric patients (age <18 years) were lower than those observed in adults, suggesting everolimus clearance (adjusted to body surface area) was higher in pediatric patients compared to adults.
Special Populations: Race
The average exposure is higher in Japanese patients compared with non-Japanese patients (Afinitor). Oral clearance is 20% higher in black patients compared with white patients.
Use: Labeled Indications
Breast cancer, advanced (Afinitor only): Treatment of advanced hormone receptor-positive, HER2-negative breast cancer in postmenopausal women (in combination with exemestane and after letrozole or anastrozole failure)
Liver transplantation (Zortress only): Prophylaxis of allograft rejection in liver transplantation (in combination with corticosteroids and reduced doses of tacrolimus, everolimus should not be administered earlier than 30 days post-transplant)
Neuroendocrine tumors (Afinitor only): Treatment of locally advanced, metastatic or unresectable progressive pancreatic neuroendocrine tumors (PNET); treatment of progressive, well-differentiated, nonfunctional GI or lung neuroendocrine tumors in patients with unresectable, locally advanced or metastatic disease
Limitations of use: Not indicated for the treatment of functional carcinoid tumors.
Renal cell carcinoma, advanced (Afinitor only): Treatment of advanced renal cell cancer (RCC) after sunitinib or sorafenib failure
Renal transplantation (Zortress only): Prophylaxis of organ rejection in renal transplant patients at low to moderate immunologic risk (in combination with basiliximab induction and concurrent with corticosteroids and reduced doses of cyclosporine)
Tuberous sclerosis complex-associated partial-onset seizures (Afinitor Disperz only): Adjunctive treatment of partial-onset seizures associated with tuberous sclerosis complex (TSC) in adult and pediatric patients ≥2 years of age
Tuberous sclerosis complex-associated renal angiomyolipoma (Afinitor only): Treatment of renal angiomyolipoma with TSC not requiring immediate surgery
Tuberous sclerosis complex-associated subependymal giant cell astrocytoma(Afinitor or Afinitor Disperz only): Treatment of subependymal giant cell astrocytoma (SEGA) associated with TSC in adults and pediatric patients ≥1 year of age which requires therapeutic intervention, but cannot be curatively resected
Use: Off Label
Carcinoid tumors (progressive, advanced)a
Data from a randomized, double-blind, placebo-controlled phase III trial in patients with low-grade or intermediate-grade neuroendocrine tumors associated with carcinoid syndrome supports the use of everolimus (in combination with octreotide LAR) for this condition Pavel 2011.
Heart transplantation (≥3 months post-transplantation) (Zortress only)b
Data from multiple studies and the ISHLT Guidelines support substitution of everolimus for mycophenolate posttransplant for prophylaxis of organ rejection in heart transplant recipients (in combination with concomitant immunosuppression). Substitution should not occur earlier than 3 months after heart transplantation due to the higher risk of rejection, as well as delayed wound healing Bara 2013, Eisen 2003, Eisen 2013, ISHLT [Costanzo 2010]. However, a recent open-label European trial demonstrated that initiation of everolimus (with reduced dose cyclosporine) within 5 days posttransplant and early calcineurin inhibitor withdrawal after 7 to 11 weeks was an effective strategy to reduce renal impairment posttransplant and maintained effectiveness in reducing the incidence of CAV Andreassen 2014, Andreassen 2016. Additionally, this approach did not show any differences in wound complications or in total surgical events Rashidi 2016. More robust trials may be necessary to further define the role of everolimus in heart transplantation. The manufacturer of everolimus (Zortress) includes a boxed warning related to increased mortality with use during the first 3 months posttransplantation and does not recommend use in heart transplantation. Clinical experience and limited clinical research data validate the use of everolimus in heart transplantation when initiation is delayed for at least 3 to 6 months posttransplantation Hollis 2015, ISHLT [Costanzo 2010].
Hodgkin lymphoma (relapsed or refractory)c
Data from a small phase II study supports the use of everolimus in the treatment of previously treated Hodgkin lymphoma in patients who had relapsed or were refractory to prior therapy Johnston 2010.
Lung transplantation (Zortress only)b
Data from 3 prospective, randomized controlled trials supports the use of everolimus starting at least 1 month post-transplant for prophylaxis of organ rejection in lung transplant recipients (in combination with concurrent corticosteroids and reduced doses of cyclosporine) Glanville 2015, Snell 2006, Strueber 2016. Additional trials may be necessary to further define the role of everolimus in this condition.
Thymoma and thymic carcinomas (advanced, refractory)b
Data from a small phase II study supports the use of everolimus in the treatment of unresectable or metastatic thymoma and thymic carcinomas after failure of at least one prior line of platinum-based therapy Zucali 2018.
Waldenström macroglobulinemia (relapsed or refractory)b
Data from a phase II trial in patients with relapsed/refractory Waldenström macroglobulinemia supports the use of everolimus for this condition Ghobrial 2010. Additional trials may be necessary to further define the role of everolimus in this condition.
Contraindications
Clinically significant hypersensitivity to everolimus, other rapamycin derivatives, or any component of the formulation
Canadian labeling: Additional contraindications (not in the US labeling): Treatment of seizures (any type) in populations other than those with a definite tuberous sclerosis complex (TSC) diagnosis
Dosage and Administration
Dosing: Adult
Note: Tablets (Afinitor, Zortress) and tablets for oral suspension (Afinitor Disperz) are not interchangeable; Afinitor Disperz is only indicated for the treatment of tuberous sclerosis complex (TSC)-associated partial-onset seizures and TSC-associated subependymal giant cell astrocytoma (SEGA), in conjunction with therapeutic monitoring. Do not combine formulations to achieve total desired dose.
Breast cancer, advanced, hormone receptor-positive, HER2-negative (Afinitor): Oral: 10 mg once daily (in combination with exemestane), continue treatment until disease progression or unacceptable toxicity
Neuroendocrine tumors (GI, lung, or pancreatic origin), advanced (Afinitor): Oral: 10 mg once daily, continue treatment until disease progression or unacceptable toxicity
Renal cell cancer, advanced (Afinitor): Oral: 10 mg once daily, continue treatment until disease progression or unacceptable toxicity
Renal cell carcinoma, advanced (off-label dose/combination): Oral: 5 mg once daily (in combination with lenvatinib), continue until disease progression or unacceptable toxicity (Motzer 2015)
Liver transplantation, rejection prophylaxis (begin at least 30 days posttransplant) (Zortress): Oral: Initial: 1 mg twice daily (in combination with tacrolimus [reduced dose required] and a corticosteroid; adjust maintenance dose if needed at a 4- to 5-day interval (from prior dose adjustment) based on serum concentrations (see Reference Range), tolerability, and response.
If trough is <3 ng/mL: Double total daily dose (using available tablet strengths)
If trough >8 ng/mL on 2 consecutive measures: Decrease dose by 0.25 mg twice daily.
Renal transplantation, rejection prophylaxis (Zortress): Oral: Initial: 0.75 mg twice daily (in combination with basiliximab induction, cyclosporine [reduced dose required] and a corticosteroid); adjust maintenance dose if needed at a 4- to 5-day interval (from prior dose adjustment) based on serum concentrations (see Reference Range), tolerability, and response.
If trough is <3 ng/mL: Double total daily dose (using available tablet strengths)
If trough >8 ng/mL on 2 consecutive measures: Decrease dose by 0.25 mg twice daily.
Tuberous sclerosis complex-associated partial-onset seizures (dosing based on body surface area [BSA]) (Afinitor Disperz): Oral: Initial: 5 mg/m2 once daily; continue until disease progression or unacceptable toxicity.
Therapeutic drug monitoring: Titrate dose to attain trough concentrations between 5 ng/mL to 15 ng/mL. Maximum dose increment at any titration must not exceed 5 mg; multiple dose titrations may be required to attain target trough concentration. Use the same assay and lab for therapeutic drug monitoring throughout treatment if possible. Adjust dose using the following equation:
New dose = Current dose x (Target concentration divided by Current concentration)
Assess trough concentrations 1 to 2 weeks after initiation of therapy, with dosage modification(s), or when changing dosage forms between tablets and tablets for oral suspension; 2 weeks after a change in hepatic function and initiation or discontinuation of concurrent CYP3A4/P-glycoprotein (P-gp) inhibitor/inducer therapy; every 3 to 6 months once stable dose is attained but BSA is changing; every 6 to 12 months once stable dose is attained and if BSA is stable throughout treatment.
Tuberous sclerosis complex-associated renal angiomyolipoma (Afinitor): Oral: 10 mg once daily, continue treatment until disease progression or unacceptable toxicity
Tuberous sclerosis complex-associated subependymal giant cell astrocytoma (SEGA; dosing based on BSA) (Afinitor or Afinitor Disperz): Oral: Initial: 4.5 mg/m2 once daily; continue until disease progression or unacceptable toxicity.
Therapeutic drug monitoring: Titrate dose to attain trough concentrations between 5 ng/mL to 15 ng/mL. Maximum dose increment at any titration must not exceed 5 mg; multiple dose titrations may be required to attain target trough concentration. Use the same assay and lab for therapeutic drug monitoring throughout treatment if possible. Adjust dose using the following equation:
New dose = Current dose x (Target concentration divided by Current concentration)
Assess trough concentrations 1 to 2 weeks after initiation of therapy, with dosage modification(s), or when changing dosage forms between tablets and tablets for oral suspension; 2 weeks after a change in hepatic function and initiation or discontinuation of concurrent CYP3A4/P-gp inhibitor/inducer therapy; every 3 to 6 months once stable dose is attained but BSA is changing; every 6 to 12 months once stable dose is attained and if BSA is stable throughout treatment.
Carcinoid tumors, advanced (off-label use): Oral: 10 mg once daily (in combination with octreotide LAR) until disease progression or toxicity (Pavel 2011)
Heart transplantation (≥3 months posttransplantation) (off-label use): Oral: Initial: 0.75 mg twice daily; adjust everolimus dose based on everolimus trough concentrations (Eisen 2003; Eisen 2013; Hollis 2015). Note: Mortality was increased with 3 mg/day when used in combination with rabbit antithymocyte globulin (Eisen 2013).
Hodgkin lymphoma, relapsed or refractory (off-label use): Oral: 10 mg once daily until disease progression or toxicity (Johnston 2010).
Lung transplantation (>1 month posttransplantation) (off-label use): Oral: Initial: 0.75 to 1.5 mg twice daily; adjust everolimus dose based on everolimus trough concentrations (Glanville 2015; Snell 2006; Strueber 2016)
Cystic fibrosis lung transplant recipients: Consider initiating with 1.25 to 2 mg twice daily since bioavailability and absorption is reduced; administer at least 30 minutes prior to a meal and with a lipase supplement (De Pablo 2013).
Thymoma and thymic carcinomas, advanced, refractory (off-label use): Oral: 10 mg once daily until disease progression or toxicity (Zucali 2018).
Waldenström macroglobulinemia, relapsed or refractory (off-label use): Oral: 10 mg once daily until disease progression or toxicity (Ghobrial 2010)
Dosage adjustment for concomitant CYP3A4 inhibitors/inducers and/or P-gp inhibitors:
Breast cancer, neuroendocrine tumors, renal cell cancer (RCC), TSC-associated renal angiomyolipoma:
CYP3A4/P-gp inducers: Strong inducers: Avoid coadministration with strong CYP3A4/P-gp inducers where alternatives exist. Avoid St. John's wort. If concomitant use cannot be avoided, double the everolimus daily dose, using increments of 5 mg or less, with careful monitoring; multiple increments may be necessary. If the strong CYP3A4/P-gp enzyme inducer is discontinued, allow 5 days to elapse prior to reducing the everolimus to the dose used prior to initiation of the CYP3A4/P-gp inducer.
CYP3A4/P-gp inhibitors:
Strong inhibitors: Avoid concomitant administration with strong CYP3A4/P-gp inhibitors. Avoid grapefruit and grapefruit juice.
Moderate CYP3A4/P-gp inhibitors: Reduce everolimus dose to 2.5 mg once daily; may consider increasing from 2.5 mg to 5 mg once daily based on patient tolerance. When the moderate inhibitor is discontinued, allow 3 days to elapse prior to adjusting the everolimus upward to the recommended starting dose or to the dose used prior to initiation of the moderate inhibitor.
Liver and renal transplantation: Dosage adjustments may be necessary based on everolimus serum concentrations
TSC-associated partial-onset seizures and SEGA:
CYP3A4/P-gp inducers: Strong inducers: Double the daily everolimus dose using increments of ≤5 mg; multiple increments may be necessary. The addition of another strong CYP3A4 inducer in a patient already receiving a strong CYP3A4 inducer may not require additional dosage modification. Assess trough concentrations when initiating and discontinuing the inducer. If the strong CYP3A4 enzyme inducer is discontinued, allow 5 days to elapse prior to reducing the everolimus to the dose used prior to initiation of the CYP3A4/P-gp inducer. Avoid St. John's wort.
CYP3A4/P-gp inhibitors:
Strong inhibitors: Avoid concomitant administration with strong CYP3A4/P-gp inhibitors. Avoid grapefruit and grapefruit juice.
Moderate CYP3A4/P-gp inhibitors:
Currently taking everolimus and starting a moderate CYP3A4/P-gp inhibitor: Reduce everolimus dose by 50%; if dose reduction is required for patients receiving the lowest strength available, administer every other day.
Discontinuing a moderate CYP3A4/P-gp inhibitor after concomitant use with everolimus: Discontinue moderate inhibitor and allow 3 days to elapse prior to resuming the everolimus dose used prior to initiation of the moderate inhibitor.
Therapeutic drug monitoring: Assess trough concentration 1 to 2 weeks after everolimus initiation or dosage modifications, or every 2 weeks with initiation or changes to concurrent CYP3A4/P-gp inhibitor therapy; maintain trough concentrations between 5 and 15 ng/mL.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Note: Tablets (Afinitor, Zortress) and/or tablets for oral suspension (Afinitor Disperz) are not interchangeable. Afinitor Disperz is indicated for the treatment of tuberous sclerosis complex (TSC)-associated subependymal giant cell astrocytoma (SEGA) or TSC-associated partial-onset seizures. Select recommended dosage form based on the indication. Do not combine formulations to achieve total desired dose.
Tuberous sclerosis complex (TSC)-associated subependymal giant cell astrocytoma (SEGA): Children ≥1 year and Adolescents: Afinitor; Afinitor Disperz: Oral: Initial: 4.5 mg/m2/dose once daily; continue until disease progression or unacceptable toxicity; evaluate serum trough concentration periodically, see TSC Therapeutic Drug Monitoring for additional information
Tuberous sclerosis complex (TSC)-associated partial-onset seizures; adjunct therapy: Children ≥2 years and Adolescents: Afinitor Disperz: Oral: Initial: 5 mg/m2/dose once daily; continue until disease progression or unacceptable toxicity; evaluate serum trough concentration periodically, see TSC Therapeutic Drug Monitoring for additional information
TSC therapeutic drug monitoring: Children ≥1 year and Adolescents: Titrate dose to attain serum trough concentrations between target range of 5 ng/mL to 15 ng/mL. Use the same assay and lab for therapeutic drug monitoring throughout treatment if possible. Adjust dose using the following equation:
New dose (mg) = Current dose (mg) x (Target concentration [ng/mL] divided by Current concentration [ng/mL])
Maximum dose increment at any titration must not exceed 5 mg; multiple dose titrations may be required to attain target trough concentration
Suggested timing of trough concentrations:
At 1 to 2 weeks following initiation, dosage modification, or switch of dosage form (tablets or tablets for oral suspension)
At 2 weeks following changes in hepatic function or modifications of concurrent CYP3A4 or p-glycoprotein (P-gp) strong inducer or moderate inhibitor therapy (initiated, dose change, or discontinued)
Every 3 to 6 months if BSA is changing and dose is stable
Every 6 to 12 months if both dose and BSA are stable
Transplantation; renal, rejection prophylaxis: Zortress: Limited data available: Children ≥1 year and Adolescents: Oral: Initial: 0.8 mg/m2/dose twice daily (maximum single dose: 1.5 mg) to maintain concentration: 3 to 6 ng/mL; reported start time of therapy variable: Within 48 hours posttransplantation was used in a multicenter, international trial of 19 pediatric patients; others have reported initiation at 2 to 4 weeks after transplantation; trials evaluating use at 3 to 4 years posttransplant in pediatric patients (<16 years) reported mean doses of 1.53 mg/m2/day and 1.3 ± 0.6 mg/m2/day; no untoward adverse effects were observed, although one center observed a higher incidence of hypertension and hyperlipidemia in the everolimus treatment group (Brunkhorst 2015; Ettenger 2008; Hoyer 2003; Pape 2007; Pape 2010; Pape 2011; Van-Damme-Lombaerts 2002; Vester 2002).
Dosage adjustment for concomitant CYP3A4 and/or P-glycoprotein (P-gp) inhibitors/inducers:
TSC-associated SEGA or partial-onset seizures: Children and Adolescents:
CYP3A4 and P-gp inducers:
Concurrent therapy: Strong inducers: Avoid concomitant administration with strong CYP3A4 inducers (including St John's wort); if concomitant use cannot be avoided, double the daily everolimus dose in increments of ≤5 mg; multiple increments may be necessary. If an additional CYP3A4 inducer is initiated (ie, 2 inducers), additional dosage adjustments may not be necessary.
Discontinuing strong CYP3A4 or P-gp inducer(s): Once all strong inducer(s) have been discontinued, allow 5 days to elapse and resume the everolimus at the dose used prior to initiation of the initial strong inducer.
Therapeutic drug monitoring: Assess everolimus serum trough concentrations when CYP3A4 or P-gp strong inducer therapy is modified (initiated, dose changed, or discontinued); see TSC Therapeutic Drug Monitoring for additional information
CYP3A4 and P-gp inhibitors:
Strong inhibitors: Avoid concomitant administration of P-gp and strong CYP3A4 inhibitors
Moderate inhibitors:
Concurrent therapy: Reduce everolimus dose by ~50%; if dose reduction is required for patients receiving the lowest strength available, consider alternate-day dosing.
Discontinuing a moderate CYP3A4 or P-gp inhibitor: Discontinue moderate inhibitor, allow 3 days to elapse and then resume the everolimus dose used prior to initiation of the moderate inhibitor.
Therapeutic drug monitoring: Assess everolimus serum trough concentrations when CYP3A4 or P-gp moderate inhibitor therapy is modified (initiated, dose changed, or discontinued); see TSC Therapeutic Drug Monitoring for additional information
Transplantation; renal: Children and Adolescents: Dosage adjustments may be necessary based on everolimus whole blood trough concentrations.
Dosing adjustment for toxicity:
Children and Adolescents:
TSC-associated SEGA or partial-onset seizures:
Nonhematologic toxicities:
Noninfectious pneumonitis:
Radiological changes suggestive of noninfectious pneumonitis but few or no symptoms: No dosage adjustment is necessary; continue treatment and monitor appropriately.
Grade 2: Interrupt treatment until symptoms improve to ≤ grade 1. Reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available. Corticosteroids may be indicated until clinical symptoms resolve. Permanently discontinue if toxicity dose not resolve or improve to grade 1 within 4 weeks.
Grade 3: Interrupt treatment until symptoms improve to ≤ grade 1. Reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available. Corticosteroids may be indicated until clinical symptoms resolve. Permanently discontinue treatment if toxicity recurs at grade 3.
Grade 4: Permanently discontinue treatment. Corticosteroids may be indicated until clinical symptoms resolve.
Stomatitis (avoid the use of products containing alcohol, hydrogen peroxide, iodine, or thyme derivatives): Note: Mouthwashes and topical treatments are recommended. Administering an alcohol-free dexamethasone oral mouthwash (swish and spit) when initiating everolimus treatment reduces the incidence and severity of stomatitis.
Grade 2: Interrupt treatment until symptoms improve to ≤ grade 1; reinitiate at same dose. If stomatitis recurs at grade 2, interrupt treatment until symptoms improve to ≤ grade 1; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 3: Interrupt treatment until symptoms improve to ≤ grade 1; then reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 4: Permanently discontinue treatment.
Metabolic toxicity (eg, hyperglycemia, dyslipidemia):
Grade 3: Temporarily interrupt treatment until symptoms improve to ≤ grade 2; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 4: Permanently discontinue treatment.
Other nonhematologic toxicities (excluding pneumonitis, stomatitis, and metabolic toxicity):
Grade 2: If toxicity becomes intolerable, temporarily interrupt treatment until improvement to ≤ grade 1 and reinitiate at the same dose; if toxicity recurs at grade 2, temporarily interrupt treatment until improvement to ≤ grade 1 and then reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 3: Temporarily interrupt treatment until improvement to ≤ grade 1; consider reinitiating treatment at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available. If toxicity recurs at grade 3, permanently discontinue treatment.
Grade 4: Permanently discontinue treatment.
Invasive systemic infection: Withhold or permanently discontinue (based on the severity of infection).
Hematologic toxicities:
Thrombocytopenia:
Grade 2 (platelets ≥50,000 to <75,000/mm3): Temporarily interrupt treatment until improvement to ≤ grade 1; reinitiate at the same dose.
Grade 3 (platelets ≥25,000 to <50,000/mm3): Temporarily interrupt treatment until improvement to ≤ grade 1; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 4 (platelets <25,000/mm3): Temporarily interrupt treatment until improvement to ≤ grade 1; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Neutropenia:
Grade 3 (ANC ≥500 to <1,000/mm3): Temporarily interrupt treatment until improvement to ≤ grade 2; reinitiate at the same dose.
Grade 4 (ANC <500/mm3): Temporarily interrupt treatment until improvement to ≤ grade 2; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Neutropenic fever:
Grade 3: Temporarily interrupt treatment until improvement to ≤ grade 2 and no fever; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 4: Permanently discontinue treatment.
Transplantation, renal:
Evidence of polyoma virus infection or PML: Consider reduced immunosuppression (taking into account the allograft risks associated with decreased immunosuppression).
Interstitial lung disease/noninfectious pneumonitis: Most cases resolve with treatment interruption (with or without corticosteroids).
Dosing: Adjustment for Toxicity
Oncology-related indications (Afinitor and Afinitor Disperz):
Noninfectious pneumonitis:
Radiological changes suggestive of non-infectious pneumonitis but few or no symptoms: No dosage adjustment is necessary; continue treatment and monitor appropriately.
Grade 2: Interrupt treatment until symptoms improve to grade 0 or 1. Reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available. Corticosteroids may be indicated until clinical symptoms resolve. Permanently discontinue if toxicity dose not resolve/improve to grade 1 within 4 weeks.
Grade 3: Interrupt treatment until symptoms improve to grade 0 or 1. Reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available. Corticosteroids may be indicated until clinical symptoms resolve. Permanently discontinue treatment if toxicity recurs at grade 3.
Grade 4: Permanently discontinue treatment. Corticosteroids may be indicated until clinical symptoms resolve.
Stomatitis (avoid the use of products containing alcohol, hydrogen peroxide, iodine, or thyme derivatives): Note: Mouthwashes and topical treatments are recommended. Administering a dexamethasone (0.5 mg/5 mL) alcohol-free oral mouthwash (10 mL swish and spit 4 times/day) when initiating everolimus treatment reduces the incidence and severity of stomatitis.
Grade 2: Interrupt treatment until symptoms improve to grade 0 or 1; reinitiate at same dose. If stomatitis recurs at grade 2, interrupt treatment until symptoms improve to grade 0 or 1; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 3: Interrupt treatment until symptoms improve to grade 0 or 1; then reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 4: Permanently discontinue treatment.
Metabolic toxicity (eg, hyperglycemia, dyslipidemia):
Grade 3: Temporarily interrupt treatment until symptoms improve to grade 0, 1, or 2; reinitiate at a 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 4: Permanently discontinue treatment.
Nonhematologic toxicities (excluding pneumonitis, stomatitis, or metabolic toxicity):
Grade 2: If toxicity becomes intolerable, temporarily interrupt treatment until improvement to grade 0 or 1 and reinitiate at the same dose; if toxicity recurs at grade 2, temporarily interrupt treatment until improvement to grade 0 or 1 and then reinitiate at a 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 3: Temporarily interrupt treatment until improvement to grade 0 or 1; consider reinitiating treatment at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available. If toxicity recurs at grade 3, permanently discontinue treatment.
Grade 4: Permanently discontinue treatment.
Invasive systemic fungal infection: Withhold or permanently discontinue (based on the severity of infection).
Hematologic toxicities:
Thrombocytopenia:
Grade 2 (platelets ≥50,000 to <75,000/mm3): Temporarily interrupt treatment until improvement to grade 0 or 1; reinitiate at the same dose.
Grade 3 (platelets ≥25,000 to <50,000/mm3): Temporarily interrupt treatment until improvement to grade 0 or 1; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 4 (platelets <25,000/mm3): Temporarily interrupt treatment until improvement to grade 0 or 1; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Neutropenia:
Grade 3 (ANC >500 to <1,000/mm3): Temporarily interrupt treatment until improvement to grade 0, 1, or 2; reinitiate at the same dose.
Grade 4 (ANC <500/mm3): Temporarily interrupt treatment until improvement to grade 0, 1, or 2; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Neutropenic fever:
Grade 3: Temporarily interrupt treatment until improvement to grade 0, 1, or 2 and no fever; reinitiate at 50% of previous dose; change to every other day dosing if the reduced dose is lower than the lowest strength available.
Grade 4: Permanently discontinue treatment.
Liver or renal transplantation (Zortress):
Evidence of polyoma virus infection or PML: Consider reduced immunosuppression (taking into account the allograft risks associated with decreased immunosuppression)
Interstitial lung disease/non-infectious pneumonitis: Most cases resolve with treatment interruption (with or without corticosteroids)
Extemporaneously Prepared
Tablets for oral suspension (Afinitor Disperz): Administer as a suspension only. Administer immediately after preparation; discard if not administered within 60 minutes after preparation. Prepare suspension in water only. Do not break or crush tablets.
Preparation in an oral syringe: Place dose into 10 mL oral syringe (maximum 10 mg/syringe; use an additional syringe for doses >10 mg). Draw ~5 mL of water and ~4 mL of air into oral syringe; allow to sit (tip up) in a container until tablets are in suspension (3 minutes). Gently invert syringe 5 times immediately prior to administration; administer contents, then add ~5 mL water and ~4 mL of air to same syringe, swirl to suspend remaining particles and administer entire contents.
Preparation in a small glass: Place dose into a small glass (≤100 mL) containing ~25 mL water (maximum 10 mg/glass; use an additional glass for doses >10 mg); allow to sit until tablets are in suspension (3 minutes). Stir gently with spoon immediately prior to administration; administer contents, then add ~25 mL water to same glass, swirl with same spoon to suspend remaining particles and administer entire contents.
Administer immediately after preparation; discard if not administered within 60 minutes after preparation.
Afinitor and Afinitor Disperz (everolimus) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; September 2017.
Tablets (Afinitor): Although the manufacturer recommends that the tablets be swallowed whole, an oral liquid may be prepared using tablets (for patients unable to swallow tablets whole). Disperse tablet in ~30 mL (1 oz) of water; gently stir. Administer and rinse container with additional 30 mL (1 oz) water and administer to ensure entire dose is administered. Administer immediately after preparation.
Afinitor (everolimus) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; July 2012.
Administration
Oral: May be taken with or without food; to reduce variability, take consistently with regard to food. Afinitor/Afinitor Disperz missed doses may be taken up to 6 hours after regularly scheduled time; if >6 hours, resume at next regularly scheduled time (do not administer double doses to make up for a missed dose).
Tablets: Swallow whole with a glass of water. Do not break, chew, or crush (do not administer tablets that are crushed or broken). Avoid contact with or exposure to crushed or broken tablets.
Tablets for oral suspension: Administer as a suspension only; wear gloves when preparing suspension. Administer immediately after preparation; discard if not administered within 60 minutes after preparation. Prepare suspension in water only. Do not break or crush tablets.
Preparation in an oral syringe: Place dose into 10 mL oral syringe (maximum: 10 mg/syringe; use an additional syringe for doses >10 mg). Draw ~5 mL of water and ~4 mL of air into oral syringe; allow to sit (tip up) in a container until tablets are in suspension (3 minutes). Gently invert syringe 5 times immediately prior to administration; administer contents, then add ~5 mL water and ~4 mL of air to same syringe, swirl to suspend remaining particles and administer entire contents.
Preparation in a small glass: Place dose into a small glass (≤100 mL) containing ~25 mL water (maximum: 10 mg/glass; use and additional glass for doses >10 mg); allow to sit until tablets are in suspension (3 minutes). Stir gently with spoon immediately prior to administration; administer contents, then add ~25 mL water to same glass, swirl with same spoon to suspend remaining particles and administer entire contents.
Breast cancer, neuroendocrine tumors, renal cell cancer, tuberous sclerosis complex (TSC)-associated partial onset-seizures, renal angiomyolipoma, and subependymal giant cell astrocytoma (SEGA): Administer at the same time each day.
Solid organ transplantation: Administer consistently ~12 hours apart; administer at the same time as cyclosporine or tacrolimus.
Dietary Considerations
Avoid grapefruit and grapefruit juice.
Storage
Tablets and tablets for suspension: Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Protect from light; protect from moisture. Store Afinitor and Afinitor Disperz in the original container. Discard suspension if not administered within 60 minutes after preparation.
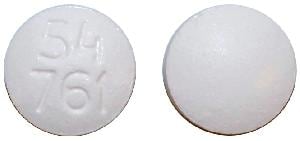
Everolimus Images
Drug Interactions
Angiotensin-Converting Enzyme Inhibitors: Everolimus may enhance the adverse/toxic effect of Angiotensin-Converting Enzyme Inhibitors. Specifically, the risk of angioedema may be increased. Monitor therapy
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antihepaciviral Combination Products: May increase the serum concentration of Everolimus. Avoid combination
ARIPiprazole: CYP3A4 Inhibitors (Weak) may increase the serum concentration of ARIPiprazole. Management: Monitor for increased aripiprazole pharmacologic effects. Aripiprazole dose adjustments may or may not be required based on concomitant therapy and/or indication. Consult full interaction monograph for specific recommendations. Monitor therapy
Baricitinib: Immunosuppressants may enhance the immunosuppressive effect of Baricitinib. Management: Use of baricitinib in combination with potent immunosuppressants such as azathioprine or cyclosporine is not recommended. Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted. Consider therapy modification
BCG (Intravesical): Immunosuppressants may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
BCG (Intravesical): Myelosuppressive Agents may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Bosentan: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Chloramphenicol (Ophthalmic): May enhance the adverse/toxic effect of Myelosuppressive Agents. Monitor therapy
Cladribine: May enhance the immunosuppressive effect of Immunosuppressants. Avoid combination
Cladribine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Avoid combination
Clofazimine: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CloZAPine: Myelosuppressive Agents may enhance the adverse/toxic effect of CloZAPine. Specifically, the risk for neutropenia may be increased. Monitor therapy
Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Monitor therapy
Conivaptan: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
CycloSPORINE (Systemic): May increase the serum concentration of Everolimus. Management: When using everolimus for renal cell carcinoma, avoid concurrent cyclosporine. When using everolimus as post-transplant immunosuppression, concurrent cyclosporine should be used at lower doses and with lower target serum cyclosporine concentrations. Consider therapy modification
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May decrease the serum concentration of Everolimus. Management: Avoid concurrent use of strong CYP3A4 inducers if possible. If coadministration cannot be avoided, double the daily dose of everolimus using increments of 5 mg or less. Monitor everolimus serum concentrations closely when indicated. Consider therapy modification
CYP3A4 Inhibitors (Moderate): May increase the serum concentration of Everolimus. Management: Everolimus dose reductions are required for most indications. See full monograph or prescribing information for specific dose adjustment and monitoring recommendations. Consider therapy modification
CYP3A4 Inhibitors (Strong): May increase the serum concentration of Everolimus. Avoid combination
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Deferiprone: Myelosuppressive Agents may enhance the neutropenic effect of Deferiprone. Management: Avoid the concomitant use of deferiprone and myelosuppressive agents whenever possible. If this combination cannot be avoided, monitor the absolute neutrophil count more closely. Consider therapy modification
Denosumab: May enhance the adverse/toxic effect of Immunosuppressants. Specifically, the risk for serious infections may be increased. Monitor therapy
Dipyrone: May enhance the adverse/toxic effect of Myelosuppressive Agents. Specifically, the risk for agranulocytosis and pancytopenia may be increased Avoid combination
Dofetilide: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Dofetilide. Monitor therapy
Echinacea: May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification
Efavirenz: May decrease the serum concentration of Everolimus. Management: Closely monitor everolimus serum concentrations when starting, stopping, or changing doses of efavirenz, particularly during the first 2 weeks after any change. Dose adjustment of everolimus may be required. Consider therapy modification
Enzalutamide: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Concurrent use of enzalutamide with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP3A4 substrate should be performed with caution and close monitoring. Consider therapy modification
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Erdafitinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Erdafitinib: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Fingolimod: Immunosuppressants may enhance the immunosuppressive effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and other immunosuppressants when possible. If combined, monitor patients closely for additive immunosuppressant effects (eg, infections). Consider therapy modification
Flibanserin: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Flibanserin. Monitor therapy
Fosaprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fusidic Acid (Systemic): May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Grapefruit Juice: May increase the serum concentration of Everolimus. Avoid combination
Idelalisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Inhibitors of CYP3A4 (Moderate) and P-glycoprotein: May increase the serum concentration of Everolimus. Management: Everolimus dose reductions are required for most indications. See full monograph or prescribing information for specific dose adjustment and monitoring recommendations. Consider therapy modification
Ivosidenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Larotrectinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Lasmiditan: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Avoid combination
Leflunomide: Immunosuppressants may enhance the adverse/toxic effect of Leflunomide. Specifically, the risk for hematologic toxicity such as pancytopenia, agranulocytosis, and/or thrombocytopenia may be increased. Management: Consider not using a leflunomide loading dose in patients receiving other immunosuppressants. Patients receiving both leflunomide and another immunosuppressant should be monitored for bone marrow suppression at least monthly. Consider therapy modification
Lemborexant: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Lemborexant. Management: The maximum recommended dosage of lemborexant is 5 mg, no more than once per night, when coadministered with weak CYP3A4 inhibitors. Consider therapy modification
Lomitapide: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Lomitapide. Management: Patients on lomitapide 5 mg/day may continue that dose. Patients taking lomitapide 10 mg/day or more should decrease the lomitapide dose by half. The lomitapide dose may then be titrated up to a max adult dose of 30 mg/day. Consider therapy modification
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
Mesalamine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Mitotane: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Doses of CYP3A4 substrates may need to be adjusted substantially when used in patients being treated with mitotane. Consider therapy modification
Natalizumab: Immunosuppressants may enhance the adverse/toxic effect of Natalizumab. Specifically, the risk of concurrent infection may be increased. Avoid combination
NiMODipine: CYP3A4 Inhibitors (Weak) may increase the serum concentration of NiMODipine. Monitor therapy
Nivolumab: Immunosuppressants may diminish the therapeutic effect of Nivolumab. Consider therapy modification
Ocrelizumab: May enhance the immunosuppressive effect of Immunosuppressants. Monitor therapy
Palbociclib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
P-glycoprotein/ABCB1 Inhibitors: May increase the serum concentration of Everolimus. Monitor therapy
Pidotimod: Immunosuppressants may diminish the therapeutic effect of Pidotimod. Monitor therapy
Pimecrolimus: May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Pimozide: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Pimozide. Avoid combination
Promazine: May enhance the myelosuppressive effect of Myelosuppressive Agents. Monitor therapy
Ranolazine: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Rifabutin: May decrease the serum concentration of Everolimus. Monitor therapy
Rifapentine: May decrease the serum concentration of Everolimus. Monitor therapy
Roflumilast: May enhance the immunosuppressive effect of Immunosuppressants. Consider therapy modification
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Simeprevir: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Siponimod: Immunosuppressants may enhance the immunosuppressive effect of Siponimod. Monitor therapy
Sipuleucel-T: Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Management: Evaluate patients to see if it is medically appropriate to reduce or discontinue therapy with immunosuppressants prior to initiating sipuleucel-T therapy. Consider therapy modification
Smallpox and Monkeypox Vaccine (Live): Immunosuppressants may diminish the therapeutic effect of Smallpox and Monkeypox Vaccine (Live). Monitor therapy
St John's Wort: May decrease the serum concentration of Everolimus. Management: Concurrent use of Afinitor brand everolimus with St Johns wort (SJW) is not recommended. Zortress brand everolimus prescribing information cautions that SJW may decrease everolimus concentrations, though no specific dose adjustment is recommended. Avoid combination
Stiripentol: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Use of stiripentol with CYP3A4 substrates that are considered to have a narrow therapeutic index should be avoided due to the increased risk for adverse effects and toxicity. Any CYP3A4 substrate used with stiripentol requires closer monitoring. Consider therapy modification
Tacrolimus (Topical): May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide. Monitor therapy
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Tofacitinib: Immunosuppressants may enhance the immunosuppressive effect of Tofacitinib. Management: Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted, and this warning seems particularly focused on more potent immunosuppressants. Consider therapy modification
Trastuzumab: May enhance the neutropenic effect of Immunosuppressants. Monitor therapy
Triazolam: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Triazolam. Management: Consider triazolam dose reduction in patients receiving concomitant weak CYP3A4 inhibitors. Consider therapy modification
Ubrogepant: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Ubrogepant. Management: In patients taking weak CYP3A4 inhibitors, the initial and second dose (if needed) of ubrogepant should be limited to 50 mg. Consider therapy modification
Upadacitinib: Immunosuppressants may enhance the immunosuppressive effect of Upadacitinib. Avoid combination
Vaccines (Inactivated): Immunosuppressants may diminish the therapeutic effect of Vaccines (Inactivated). Management: Vaccine efficacy may be reduced. Complete all age-appropriate vaccinations at least 2 weeks prior to starting an immunosuppressant. If vaccinated during immunosuppressant therapy, revaccinate at least 3 months after immunosuppressant discontinuation. Consider therapy modification
Vaccines (Live): Immunosuppressants may enhance the adverse/toxic effect of Vaccines (Live). Immunosuppressants may diminish the therapeutic effect of Vaccines (Live). Management: Avoid use of live organism vaccines with immunosuppressants; live-attenuated vaccines should not be given for at least 3 months after immunosuppressants. Exceptions: Smallpox and Monkeypox Vaccine (Live). Avoid combination
Venetoclax: May increase the serum concentration of Everolimus. Management: Administer everolimus at least 6 hours before venetoclax when concomitant therapy is required. Consider therapy modification
Voriconazole: May increase the serum concentration of Everolimus. Avoid combination
Adverse Reactions
Transplantation:
Reactions occur in kidney and liver transplantation unless otherwise specified.
>10%:
Cardiovascular: Peripheral edema (kidney transplant: 45%; liver transplant: 18% to 20%), hypertension (17% to 30%)
Central nervous system: Headache (18% to 22%), insomnia (kidney transplant: 17%; liver transplant: 6% to 7%), procedural pain (kidney transplant: 15%), fatigue (9% to 11%)
Endocrine & metabolic: Diabetes mellitus (new onset: liver transplant: 32%, kidney transplant: 9%), hyperkalemia (renal transplant: 18%), hypercholesterolemia (9% to 17%), hypomagnesemia (kidney transplant: 14%), hypophosphatemia (kidney transplant: 13%), hyperglycemia (kidney transplant: 12%), hypokalemia (kidney transplant: 12%)
Gastrointestinal: Constipation (kidney transplant: 38%), nausea (kidney transplant: 29%; liver transplant: 14% to 15%), diarrhea (19% to 24%), vomiting (kidney transplant: 15%), abdominal pain (13% to 15%)
Genitourinary: Urinary tract infection (kidney transplant: 22%), hematuria (kidney transplant: 12%), dysuria (kidney transplant: 11%)
Hematologic & oncologic: Anemia (kidney transplant: 26%), leukopenia (3% to 13%)
Infection: Infection (kidney transplant: 62%; liver transplant: 50%), viral infection (liver transplant: 17%; kidney transplant: 10%), bacterial infection (liver transplant: 16%), hepatitis C (liver transplant: 11% to 14%)
Local: Incisional pain (kidney transplant: 16%)
Neuromuscular & skeletal: Limb pain (kidney transplant: 12%), back pain (kidney transplant: 11%)
Renal: Increased serum creatinine (kidney transplant: 18%)
Respiratory: Upper respiratory tract infection (kidney transplant: 16%)
Miscellaneous: Postoperative wound complication (kidney transplant: 35%; liver transplant: 11%; includes incisional hernia, lymphocele, seroma, wound dehiscence), fever (13% to 19%)
1% to 10%:
Cardiovascular: Hypertensive crisis (1%), angina pectoris, atrial fibrillation, cardiac failure, chest discomfort, chest pain, deep vein thrombosis, edema, hypotension, palpitations, phlebitis, pulmonary embolism, renal artery thrombosis, syncope, tachycardia, venous thromboembolism
Central nervous system: Agitation, anxiety, chills, depression, dizziness, drowsiness, hallucination, hemiparesis, hypoesthesia, lethargy, malaise, migraine, myasthenia, neuralgia, pain, paresthesia
Dermatologic: Acne vulgaris, acneiform eruption, alopecia, cellulitis, diaphoresis, ecchymoses, folliculitis, hypertrichosis, night sweats, onychomycosis, pruritus, skin rash, tinea pedis
Endocrine & metabolic: Acidosis, amenorrhea, cushingoid appearance, cyanocobalamin deficiency, dehydration, fluid retention, gout, hirsutism, hypercalcemia, hyperparathyroidism, hypertriglyceridemia, hyperuricemia, hypocalcemia, hypoglycemia, hyponatremia, hypothyroidism, iron deficiency, ovarian cyst
Gastrointestinal: Stomatitis (kidney transplant: 8%), dyspepsia (kidney transplant: 4%), upper abdominal pain (kidney transplant: 3%), abdominal distention, anorexia, biliary obstruction, cholangitis, cholestasis, decreased appetite, dysphagia, epigastric distress, flatulence, gastritis, gastroenteritis, gastroesophageal reflux disease, gingival hyperplasia, hematemesis, hemorrhoids, hernia of abdominal cavity, inguinal hernia, intestinal obstruction, oral candidiasis, oral herpes simplex infection, oral mucosa ulcer, peritoneal effusion, peritonitis
Genitourinary: Erectile dysfunction (kidney transplant: 5%), benign prostatic hyperplasia, bladder spasm, nocturia, perinephric abscess, perinephric hematoma, pollakiuria, proteinuria, pyuria, scrotal edema, urethritis, urinary retention, urinary urgency
Hematologic & oncologic: Neoplasm (3% to 4%), leukocytosis, lymphadenopathy, lymphorrhea, neutropenia, pancytopenia, thrombocythemia, thrombocytopenia
Hepatic: Abnormal hepatic function tests (liver transplant: 7% to 8%), ascites (liver transplant: 4%), hepatitis (noninfectious), increased liver enzymes, increased serum alkaline phosphatase, increased serum bilirubin
Hypersensitivity: Angioedema (<1%)
Infection: BK virus (kidney transplant: 1%), bacteremia, candidiasis, cytomegalovirus disease, herpes virus infection, influenza, sepsis, wound infection
Neuromuscular & skeletal: Tremor (8% to 10%), arthralgia, asthenia, joint swelling, muscle spasm, musculoskeletal pain, myalgia, osteoarthritis, osteomyelitis, osteonecrosis, osteoporosis, spondylitis
Ophthalmic: Blurred vision, cataract, conjunctivitis
Renal: Renal failure syndrome (5% to 10%; may be acute), hydronephrosis, increased blood urea nitrogen, interstitial nephritis, polyuria, pyelonephritis, renal insufficiency, renal tubular necrosis
Respiratory: Cough (kidney transplant: 7%), atelectasis, bronchitis, dyspnea, epistaxis, lower respiratory tract infection, nasal congestion, nasopharyngitis, oropharyngeal pain, paranasal sinus congestion, pleural effusion, pneumonia, pulmonary edema, rhinorrhea, sinusitis, wheezing
Antineoplastic:
Antineoplastic indications include advanced hormone receptor-positive, advanced nonfunctional NET of gastrointestinal or lung origin, HER2-negative breast cancer, pancreatic neuroendocrine tumors, renal cell carcinoma, and tuberous sclerosis complex associated renal angiomyolipoma, subependymal giant cell astrocytoma, or seizures
>10%:
Cardiovascular: Edema (≤39%), peripheral edema (≤39%), hypertension (4% to 13%)
Central nervous system: Fatigue ( ≤45%), malaise (≤45%), headache (≤30%), migraine (≤30%), behavioral problems (21%; includes abnormal behavior, agitation, anxiety, obsessive compulsive symptoms, panic attack), insomnia (6% to 14%), dizziness (7% to 12%)
Dermatologic: Skin rash (6% to 59%), cellulitis (29%), acne vulgaris (10% to 22%), nail disease (5% to 22%), pruritus (13% to 21%), xeroderma (13%)
Endocrine & metabolic: Hypercholesterolemia (71% to 86%), hyperglycemia (14% to 75%), hypertriglyceridemia (27% to 73%), decreased serum bicarbonate (56%), hypophosphatemia (9% to 49%), decreased serum calcium (37%), hypokalemia (27%), hypoalbuminemia (18%), amenorrhea (15% to 17%)
Gastrointestinal: Stomatitis (9% to 78%), diarrhea (14% to 50%), abdominal pain (5% to 36%), decreased appetite (6% to 30%), vomiting (10% to 29%), nausea (8% to 29%), weight loss (5% to 28%), anorexia (25%), dysgeusia (5% to 22%), mucositis (19%), constipation (10% to 14%), xerostomia (8% to 11%)
Genitourinary: Urinary tract infection (5% to 16%), irregular menses (10% to 11%)
Hematologic & oncologic: Anemia (27% to 81%; grades 3/4: 5% to 15%), increase in fasting plasma glucose (14% to 75%, grades 3/4: 17%), prolonged partial thromboplastin time (72%; grades 3/4: 3%), lymphocytopenia (20% to 66%, grades 3/4: 1% to 18%), leukopenia (37% to 49%; grades 3/4: 2%), neutropenia (25% to 46%, grades 3/4: ≤9%), thrombocytopenia (12% to 33%; grades 3/4: 1% to 3%)
Hepatic: Increased serum alkaline phosphatase (32% to 74%), increased serum aspartate aminotransferase (13% to 69%), increased serum alanine aminotransaminase (17% to 51%)
Infection: Infection (37% to 58%)
Neuromuscular & skeletal: Asthenia (13% to 33%), arthralgia (13% to 20%), back pain (14% to 15%), limb pain (8% to 14%)
Renal: Increased serum creatinine (19% to 50%)
Respiratory: Respiratory tract infection (31%), cough (10% to 30%; includes productive cough), nasopharyngitis (≤25%), rhinitis (≤25%), upper respiratory tract infection (≤25%), dyspnea (20% to 24%; includes dyspnea on exertion), epistaxis (5% to 22%), pneumonitis (≤19%; may include interstitial pulmonary disease, pulmonary alveolar hemorrhage, pulmonary alveolitis, pulmonary fibrosis, pulmonary infiltrates, pulmonary toxicity, restrictive pulmonary disease), oropharyngeal pain (11%)
Miscellaneous: Fever (14% to 31%)
1% to 10%:
Cardiovascular: Chest pain (5%), tachycardia (3%), cardiac failure (1%), deep vein thrombosis (<1%)
Central nervous system: Depression (5%), paresthesia (5%), chills (4%), aggressive behavior (≤2%)
Dermatologic: Alopecia (10%), palmar-plantar erythrodysesthesia (5%), erythema (4%), onychoclasis (4%), skin lesion (4%), acneiform eruption (3%)
Endocrine & metabolic: Diabetes mellitus (10%; new onset: <1%), heavy menstrual bleeding (6% to 10%), menstrual disease (6% to 10%), decreased serum fibrinogen (8%), increased luteinizing hormone (1% to 4%), increased follicle-stimulating hormone (3%), ovarian cyst (≤3%), exacerbation of diabetes mellitus (2%)
Gastrointestinal: Gastroenteritis (10%), hemorrhoids (5%), dysphagia (4%)
Genitourinary: Vaginal hemorrhage (8%), dysmenorrhea (6%), uterine hemorrhage (6%), cystitis (3%), proteinuria (2%)
Hematologic & oncologic: Hemorrhage (3%)
Hepatic: Increased serum bilirubin (3%)
Hypersensitivity: Hypersensitivity reaction (≤3%; includes anaphylaxis, chest pain, dyspnea, flushing), angioedema (≤1%)
Infection: Candidiasis (<1%), hepatitis C (<1%), sepsis (<1%)
Neuromuscular & skeletal: Muscle spasm (10%), jaw pain (3%)
Ophthalmic: Eyelid edema (4%), conjunctivitis (2%)
Otic: Otitis media (6%)
Renal: Renal failure syndrome (3%)
Respiratory: Streptococcal pharyngitis (10%), pleural effusion (7%), pneumonia (2% to 6%), bronchitis (4%), pharyngolaryngeal pain (4%), rhinorrhea (3%), sinusitis (3%)
Miscellaneous: Postoperative wound complication (<1%; wound healing impairment)
<1%, postmarketing, and/or case reports: Arterial thrombosis, aspergillosis, azoospermia, cholecystitis, cholelithiasis, complex regional pain syndrome, decreased plasma testosterone, hypersensitivity angiitis, male infertility, nephrotoxicity, oligospermia, pancreatitis (including acute pancreatitis), pericardial effusion, polyoma virus infection, progressive multifocal leukoencephalopathy, reactivation of HBV, respiratory distress, septic shock, thrombosis of vascular graft (kidney), thrombotic microangiopathy
Warnings/Precautions
Concerns related to adverse effects:
- Angioedema: Everolimus is associated with the development of angioedema; concomitant use with other agents known to cause angioedema (eg, ACE inhibitors) may increase the risk. Permanently discontinue if angioedema occurs.
- Bone marrow suppression: Decreases in hemoglobin, neutrophils, platelets, and lymphocytes have been reported, including grade 3 and 4 events. Monitor blood counts at baseline, every 6 months for the first year of treatment, and annually thereafter. Withhold or permanently discontinue treatment based on severity.
- Edema: Generalized edema (including peripheral edema and lymphedema) and local fluid accumulation (eg, pericardial effusion, pleural effusion, ascites) may occur.
- Graft thrombosis: [US Boxed Warning]: An increased risk of renal arterial and venous thrombosis has been reported with use in renal transplantation, generally within the first 30 days after transplant; may result in graft loss.
- Hepatic artery thrombosis: MTOR inhibitors are associated with an increase in hepatic artery thrombosis, most cases have been reported within 30 days after transplant and usually proceeded to graft loss or death. Do not use everolimus prior to 30 days post liver transplant.
- Hypersensitivity: Severe hypersensitivity reactions (anaphylaxis, dyspnea, flushing, chest pain, and angioedema) have been reported. Permanently discontinue use if clinically significant hypersensitivity occurs.
- Infections: [US Boxed Warning]: Everolimus has immunosuppressant properties which may result in increased susceptibility to infection. Everolimus may predispose patients to bacterial, fungal, viral, or protozoal infections, including opportunistic infections. Polyoma virus infection in transplant patients may be serious and/or fatal. Polyoma virus-associated nephropathy (primarily due to BK virus), which may result in serious cases of deteriorating renal function and renal graft loss, has been observed with use in renal transplantation. JC virus-associated progressive multiple leukoencephalopathy (PML) may also be associated with everolimus use in transplantation. Reduced immunosuppression (taking into account the risks of rejection) should be considered with evidence of polyoma virus infection or PML. Localized and systemic infections (including pneumonia, mycobacterial infections, other bacterial infections, invasive fungal infections [eg, aspergillosis, candidiasis, or Pneumocystis jirovecii pneumonia {PCP}] and viral infections [eg, hepatitis B reactivation]) have occurred. Grade 3 and 4 infections have been reported; some infections have been severe (eg, sepsis, septic shock, or resulting in multisystem organ failure) or fatal. Serious infections have been reported at a higher frequency in patients <6 years of age. Treatment of preexisting invasive fungal infections should be completed prior to starting everolimus treatment. Monitor for signs and symptoms of infection. Withhold or permanently discontinue everolimus based on severity of infection. When concomitant corticosteroids or other immunosuppressive agents are required, administer PCP prophylaxis. Combination immunosuppressant therapy in solid organ transplantation should be used with caution due to the risk of over immunosuppression, which may lead to increased susceptibility to infection. Antimicrobial prophylaxis for PCP and prophylaxis for cytomegalovirus (CMV) is recommended in transplant recipients.
- Malignancy: [US Boxed Warning]: Immunosuppressant use may result in the development of malignancy, including lymphoma and skin cancer. The risk is associated with treatment intensity and the duration of therapy. To minimize the risk for skin cancer, limit exposure to sunlight and ultraviolet light; wear protective clothing, and use effective sunscreen.
- Metabolic effects: Hyperglycemia, hyperlipidemia, and hypertriglyceridemia have been reported, including grade 3 and 4 events. Higher serum everolimus concentrations are associated with an increased risk for hyperlipidemia. Everolimus has not been studied in transplant patients with baseline cholesterol >350 mg/dL. Monitor fasting glucose and lipid profile prior to treatment initiation and annually thereafter. Monitor more frequently in patients with concomitant medications affecting glucose. Increases in serum glucose are common; may alter insulin and/or oral hypoglycemic therapy requirements in patients with diabetes. The risk for new-onset diabetes is increased with everolimus use after transplantation. Manage with appropriate medical therapy (if possible, optimize glucose control and lipids prior to treatment initiation). Antihyperlipidemic therapy may not normalize levels. Based on the severity, metabolic events may require treatment interruption or discontinuation.
- Mucositis/stomatitis: Everolimus is commonly associated with mouth ulcers, mucositis, and stomatitis. Stomatitis typically occurs within the first 8 weeks of therapy. When used for oncology indications, dexamethasone 0.5 mg/5 mL alcohol-free oral solution mouthwash (10 mL swish and spit 4 times/day) reduces the incidence and severity of stomatitis if initiated at the onset of everolimus treatment (avoid food or drink within 1 hour after dexamethasone). If stomatitis does occur, manage with mouthwash and/or topical therapy; avoid the use of alcohol-, hydrogen peroxide-, iodine-, or thyme-based products. Due to the high potential for drug interactions, avoid the use of systemic antifungals unless fungal infection has been diagnosed.
- Nephrotoxicity: Elevations in serum creatinine (generally mild), renal failure, and proteinuria have been observed with everolimus. Grade 3 and 4 serum creatinine elevations and proteinuria have been reported. Monitor renal function (BUN, creatinine, and/or urinary protein). Risk of nephrotoxicity may be increased when administered with calcineurin inhibitors (eg, cyclosporine, tacrolimus); dosage adjustment of calcineurin inhibitor is necessary. Monitor for proteinuria; the risk of proteinuria is increased when everolimus is used in combination with cyclosporine, and with higher serum everolimus concentrations.
- Pulmonary toxicity: Noninfectious pneumonitis, interstitial lung disease (ILD), and/or noninfectious fibrosis have been observed with mTOR inhibitors, including everolimus; some cases were fatal. Promptly evaluate worsening respiratory symptoms. Cases of ILD have been reported with pulmonary hypertension (including pulmonary arterial hypertension) as a secondary event. Consider opportunistic infections such as PCP when evaluating clinical symptoms. Everolimus treatment may be continued (without dose adjustment in patients who develop radiological changes suggestive of non-infectious pneumonitis with few or no symptoms [imaging appears to overestimate the incidence of clinical pneumonitis]). Based on the severity, withhold or permanently discontinue everolimus for grade 2 to 4 non-infectious pneumonitis; corticosteroids may be indicated until resolution of clinical symptoms. When concomitant corticosteroids or other immunosuppressive agents are required, administer PCP prophylaxis. Pneumonitis has been reported even at reduced everolimus doses.
- Wound healing complications: Everolimus may delay wound healing and increase the occurrence of wound-related complications (eg, wound dehiscence, infection, incisional hernia, lymphocele, seroma); may require surgical intervention. Use everolimus with caution in the peri-surgical period.
Disease-related concerns:
- Heart transplantation: [US Boxed Warning]: Increased mortality (usually associated with infections) within the first 3 months after transplant was noted in a study of patients with de novo heart transplant receiving immunosuppressive regimens containing everolimus (with or without induction therapy). Use in heart transplantation is not recommended. The boxed warning in the labeling (Zortress) is based on severe infectious complications, rather than efficacy (reduction in the incidence of cardiac allograft vasculopathy). Despite labeled warnings for this off-label indication, some centers continue to use everolimus (with reduced calcineurin inhibitor exposure). However, everolimus initiation in heart transplantation is delayed until 3 to 6 months post-transplantation due to impaired wound healing and pericardial effusions early on in the postoperative period (Andreassen 2014; Andreassen 2016; Costanzo 2010; Hirt 2013; Hollis 2015).
- Hepatic impairment: Everolimus exposure is increased in patients with hepatic impairment. For patients with breast cancer, neuroendocrine tumors, renal cell cancer (RCC), or tuberous sclerosis complex (TSC)-associated renal angiomyolipoma with mild and moderate hepatic impairment, reduced doses are recommended; in patients with severe hepatic impairment, use is recommended (at reduced doses) if the potential benefit outweighs risks. Reduced doses are recommended in transplant patients with hepatic impairment (Child-Pugh class A, B, or C); monitor whole blood trough levels closely. For patients with TSC-associated partial onset seizures and subependymal giant cell astrocytoma (SEGA), reduced doses are recommended in severe hepatic impairment; monitor whole blood trough levels.
- Hereditary galactose intolerance: Avoid use in patients with galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption; may result in diarrhea and malabsorption.
- Renal impairment: An increased incidence of rash, infection and dose interruptions have been reported in patients with renal insufficiency (CrCl ≤60 mL/minute) who received mTOR inhibitors for the treatment of renal cell cancer (Gupta 2011). Serum creatinine elevations and proteinuria have been reported. Monitor renal function (BUN, serum creatinine, urinary protein) at baseline and periodically, especially if risk factors for further impairment exist. Pharmacokinetic studies have not been conducted; dosage adjustments are not required based on renal impairment.
- Transplantation (solid organ): The safety and efficacy of everolimus in renal transplantation patients with high-immunologic risk or in solid organ transplant other than renal or liver have not been established. In liver transplant, tacrolimus has minimal or no pharmacokinetic impact on everolimus concentrations.
Concurrent drug therapy issues:
- Calcineurin inhibitor combination therapy: [US Boxed Warning]: Due to the increased risk for nephrotoxicity in renal transplantation, avoid standard doses of cyclosporine in combination with everolimus; reduced cyclosporine doses are recommended when everolimus is used in combination with cyclosporine. Therapeutic monitoring of cyclosporine and everolimus concentrations is recommended. Everolimus and cyclosporine combination therapy may result in increased proteinuria and may increase the risk for thrombotic microangiopathy/thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TMA/TTP/HUS); monitor blood counts. In liver transplantation, the tacrolimus dose and target range should be reduced to minimize the risk of nephrotoxicity. Eliminating calcineurin inhibitors from the immunosuppressive regimen may result in acute rejection.
- Drug-drug/drug-food interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
- HMG-CoA reductase inhibitors: In transplant patients, avoid the use of certain HMG-CoA reductase inhibitors (eg, simvastatin, lovastatin); may increase the risk for rhabdomyolysis due to the potential interaction with cyclosporine (which may be given in combination with everolimus for transplantation).
Dosage form specific issues:
- Tablet formulations: Tablets (Afinitor, Zortress) and tablets for oral suspension (Afinitor Disperz) are not interchangeable; Afinitor Disperz is only indicated in conjunction with therapeutic monitoring for the treatment of tuberous sclerosis complex-associated partial-onset seizures and SEGA. Do not combine formulations to achieve total desired dose.
Other warnings/precautions:
- Assay method: For indications requiring whole blood trough concentrations to determine dosage adjustments, a consistent method should be used; concentration values from different assay methods may not be interchangeable.
- Experienced physician: [US Boxed Warning]: In transplantation, everolimus should only be used by physicians experienced in immunosuppressive therapy and management of transplant patients. Adequate laboratory and supportive medical resources must be readily available.
- Immunizations: Patients should not be immunized with live viral vaccines during or shortly after treatment and should avoid close contact with recently vaccinated (live vaccine) individuals. In pediatric patients, complete recommended series of live virus childhood vaccinations prior to everolimus treatment (if immediate everolimus treatment is not indicated); an accelerated vaccination schedule may be appropriate.
Monitoring Parameters
CBC with differential (baseline, every 6 months during the first year of treatment, and then annually); liver function (baseline and periodic); serum creatinine (baseline and periodic), urinary protein (baseline and periodic), and BUN (baseline and periodic); fasting serum glucose (baseline and annually in nondiabetic patients and more frequently as indicated in diabetic patients), HbA1c, and lipid profile (baseline and annually thereafter); monitor for signs and symptoms of infection, noninfectious pneumonitis, stomatitis, or malignancy; renal function every 6 months (patients with additional risk factors for renal failure); pregnancy test (in females of reproductive potential prior to initiating therapy). Monitor adherence.
Solid organ transplantation: Monitor everolimus whole blood trough concentrations (based on an LC/MS/MS assay method), especially in patients with hepatic impairment, with concomitant CYP3A4 inhibitors and inducers, and when cyclosporine or tacrolimus formulations or doses are changed; dosage adjustments should be made on trough concentrations obtained 4 to 5 days after a previous dosage adjustment; monitor cyclosporine or tacrolimus concentrations; monitor for proteinuria
Tuberous sclerosis complex (TSC)-associated partial onset seizures and subependymal giant cell astrocytoma (SEGA): Monitor everolimus whole blood trough concentrations 1 to 2 weeks after treatment initiation, with dosage modification(s), or when changing dosage forms between tablets and tablets for oral suspension, 2 weeks after a change in hepatic function and initiation or discontinuation of concurrent CYP3A4/P-glycoprotein (P-gp) inhibitor/inducer therapy. Maintain trough concentrations between 5 and 15 ng/mL; once stable dose is attained and if body surface area (BSA) is stable throughout treatment, monitor trough concentrations every 6 to 12 months (monitor every 3 to 6 months if BSA is changing).
Pregnancy
Pregnancy Considerations
Based on the mechanism of action and data from animal reproduction studies, everolimus may cause fetal harm if administered during pregnancy. Information related to the use of everolimus in pregnancy is limited (Yamamura 2017).
Verify pregnancy status in females of reproductive potential prior to initiating therapy. Females of reproductive potential should be advised to avoid pregnancy and use highly effective birth control during treatment and for 8 weeks after the last everolimus dose. Male patients with female partners of reproductive potential should use effective contraception during treatment and for 4 weeks after the last everolimus dose.
Everolimus may cause infertility. In females, menstrual irregularities, secondary amenorrhea, and increases in luteinizing hormone and follicle-stimulating hormone have occurred. Azoospermia and oligospermia have been observed in males.
The Transplant Pregnancy Registry International (TPR) is a registry that follows pregnancies that occur in maternal transplant recipients or those fathered by male transplant recipients. The TPR encourages reporting of pregnancies following solid organ transplant by contacting them at 1-877-955-6877 or https://www.transplantpregnancyregistry.org.
Patient Education
What is this drug used for?
- It is used to treat cancer.
- It is used to treat certain kinds of kidney cysts.
- It is used to keep the body from harming the organ after an organ transplant.
- It is used to help control certain kinds of seizures.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Acne
- Trouble sleeping
- Headache
- Loss of strength and energy
- Diarrhea
- Constipation
- Change in taste
- Stuffy nose
- Sore throat
- Lack of appetite
- Dry mouth
- Mouth sores
- Mouth irritation
- Dry skin
- Nail changes
- Hair loss
- Joint pain
- Painful extremities
- Muscle spasms
- Nosebleed
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- High blood sugar like confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit.
- Infection
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin
- Severe pulmonary disorder like lung or breathing problems like difficulty breathing, shortness of breath, or a cough that is new or worse
- Kidney problems like unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain
- Redness or irritation of palms or soles of feet
- Burning or numbness feeling
- Flushing
- Chest pain
- Bruising
- Bleeding
- Seizures
- Fast heartbeat
- Abnormal heartbeat
- Severe dizziness
- Passing out
- Muscle pain
- Muscle weakness
- Menstrual changes
- Mood changes
- Behavioral changes
- Edema
- Mole changes
- Skin growths
- Swollen glands
- Night sweats
- Excessive weight loss
- Abdominal pain
- Back pain
- Groin pain
- Severe nausea
- Vomiting
- Progressive multifocal leukoencephalopathy like confusion, depression, trouble with memory, behavioral changes, change in strength on one side is greater than the other, difficulty speaking, change in balance, or vision changes
- Severe wound irritation, pus, or pain
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.