Boxed Warning
Cardiovascular death:
Gout patients with established cardiovascular (CV) disease treated with febuxostat had a higher rate of CV death compared to those treated with allopurinol in a CV outcomes study. Consider the risks and benefits of febuxostat when deciding to prescribe or continue patients on febuxostat. Febuxostat should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Uloric: 40 mg, 80 mg
Generic: 40 mg, 80 mg
Pharmacology
Mechanism of Action
Selectively inhibits xanthine oxidase, the enzyme responsible for the conversion of hypoxanthine to xanthine to uric acid thereby decreasing uric acid. At therapeutic concentration does not inhibit other enzymes involved in purine and pyrimidine synthesis.
Pharmacokinetics/Pharmacodynamics
Absorption
≥49%
Distribution
Vss: ~50 L
Metabolism
Extensive conjugation via uridine diphosphate glucuronosyltransferases (UGTs) 1A1, 1A3, 1A9, and 2B7 and oxidation via cytochrome P450 (CYP) 1A2, 2C8, and 2C9 as well as non-P450 enzymes. Oxidation leads to formation of active metabolites (67M-1, 67M-2, 67M-4)
Excretion
Urine (~49% mostly as metabolites, 3% as unchanged drug); feces (~45% mostly as metabolites, 12% as unchanged drug)
Time to Peak
Plasma: 1 to 1.5 hours
Half-Life Elimination
~5 to 8 hours
Protein Binding
~99%, primarily to albumin
Use in Specific Populations
Special Populations: Gender
Following multiple oral doses, Cmax and AUC are 30% and 14% higher in women than men, respectively.
Use: Labeled Indications
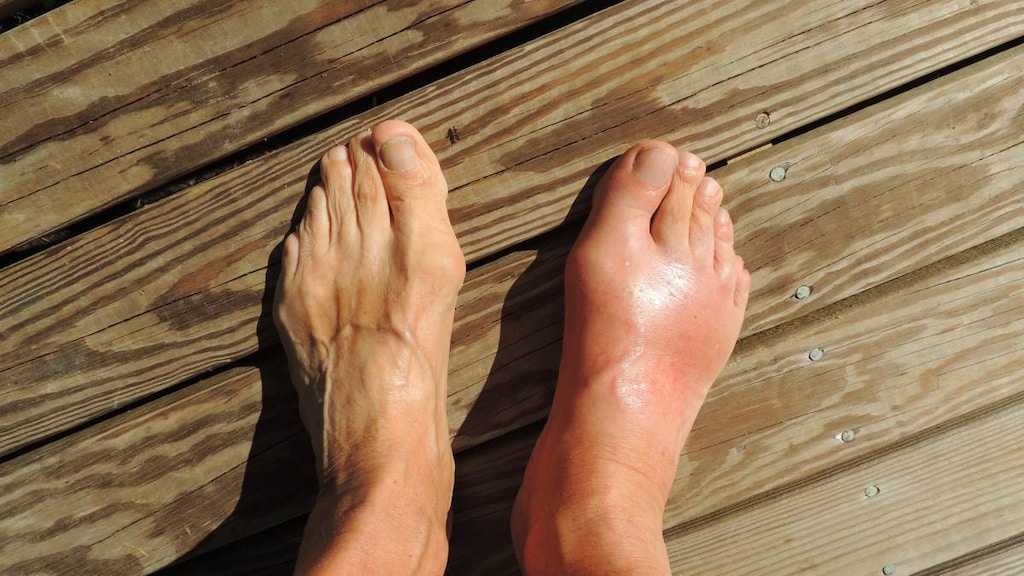
Hyperuricemia: Chronic management of hyperuricemia in patients with gout who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or for whom treatment with allopurinol is not advisable
Limitations of use: Not recommended for treatment of asymptomatic hyperuricemia.
Contraindications
Concurrent use with azathioprine or mercaptopurine
Canadian labeling: Additional contraindications (not in US labeling): Hypersensitivity to febuxostat or any component of the formulation.
Dosage and Administration
Dosing: Adult
Note: It is recommended to take an NSAID or colchicine with initiation of therapy and may continue for up to 6 months to help prevent gout flares. If a gout flare occurs, febuxostat does not need to be discontinued.
Hyperuricemia: Oral: Initial: 40 mg once daily; may increase to 80 mg once daily in patients who do not achieve a serum uric acid level <6 mg/dL after 2 weeks. The dose may be increased further to 120 mg once daily if clinically indicated (ACR guidelines [Khanna 2012]; EULAR [Richette 2017]).
Dosing: Geriatric
Refer to adult dosing.
Administration
Administer with or without meals or antacids.
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Protect from light.
Febuxostat Images
Drug Interactions
AzaTHIOprine: Febuxostat may increase the serum concentration of AzaTHIOprine. Avoid combination
Didanosine: Febuxostat may increase the serum concentration of Didanosine. Avoid combination
Mercaptopurine: Febuxostat may increase the serum concentration of Mercaptopurine. Avoid combination
Pegloticase: Febuxostat may enhance the adverse/toxic effect of Pegloticase. Specifically, Febuxostat may blunt increases in serum urate that would signal an elevated risk of anaphylaxis and infusion reactions. Avoid combination
Theophylline Derivatives: Febuxostat may increase serum concentrations of the active metabolite(s) of Theophylline Derivatives. Specifically, concentrations of 1-methylxanthine, a metabolite of unknown clinical importance, may become elevated. Exceptions: Dyphylline. Monitor therapy
Adverse Reactions
1% to 10%:
Dermatologic: Skin rash (2%)
Gastrointestinal: Nausea (1%)
Hepatic: Hepatic insufficiency (5% to 7%), increased serum alanine aminotransferase (3%), increased serum aspartate aminotransferase (2%)
Neuromuscular & skeletal: Arthralgia (≤1%)
Frequency not defined: Endocrine & metabolic: Acute gout attack
<1%, postmarketing, and/or case reports: Abdominal distention, abdominal pain, abnormal electroencephalogram, abnormal gait, abnormal hepatic function tests, abnormal skin odor, aggressive behavior, agitation, agranulocytosis, alopecia, altered sense of smell, anaphylaxis, anemia, angina pectoris, angioedema, anorexia, anxiety, arthritis, asthenia, atrial fibrillation, atrial flutter, blood coagulation test abnormality, blurred vision, bronchitis, bruise, casts in urine, cerebral infarction (lacunar), cerebrovascular accident, chest discomfort, chest pain, cholecystitis, cholelithiasis, constipation, cough, deafness, decreased appetite, decreased libido, decreased mental acuity, decreased serum bicarbonate, decreased urine output, dehydration, depression, dermatitis, diabetes mellitus, drowsiness, drug reaction with eosinophilia and systemic symptoms, dry nose, dysgeusia, dyspepsia, dyspnea, ecchymoses, ECG abnormality, eczema, edema, eosinophilia, epistaxis, equilibrium disturbance, erectile dysfunction, erythema multiforme, exfoliation of skin, fatigue, feeling abnormal, flatulence, flu-like symptoms, flushing, frequent bowel movements, gastric hyperacidity, gastritis, gastroesophageal reflux disease, gastrointestinal distress, gingival pain, Guillain-Barré syndrome, gynecomastia, hair discoloration, headache, heart murmur, hematemesis, hematochezia, hematuria, hemiparesis, hepatic disease, hepatic failure, hepatitis, hepatomegaly, herpes zoster, hirsutism, hot flash, hypercholesterolemia, hyperglycemia, hyperhidrosis, hyperlipidemia, hypersensitivity reaction, hypertension, hypertonia, hypertriglyceridemia, hypoesthesia, hypokalemia, hypotension, immune thrombocytopenia, increased amylase, increased appetite, increased blood urea nitrogen, increased creatine phosphokinase in blood specimen, increased lactate dehydrogenase, increased LDL cholesterol, increased MCV, increased serum alkaline phosphatase, increased serum creatinine, increased serum glucose, increased serum potassium, increased serum sodium, increased thirst, increased thyroid stimulating hormone level, increased urine output, insomnia, interstitial nephritis, irritability, jaundice, joint stiffness, joint swelling, lethargy, leukocytosis, leukocyturia, leukopenia, liver steatosis, lymphocytopenia, mass, mastalgia, migraine, muscle rigidity, muscle spasm, muscle twitching, musculoskeletal pain, myalgia, myasthenia, nephrolithiasis, nervousness, neutropenia, oral mucosa ulcer, pain, palpitations, pancreatitis, pancytopenia, panic attack, paranasal sinus hypersecretion, paresthesia, peptic ulcer, personality changes, petechia, pharyngeal edema, pollakiuria, prolonged partial thromboplastin time, prolonged prothrombin time, prostate specific antigen increase, proteinuria, pruritus, psychotic symptoms, purpuric disease, renal failure syndrome, renal insufficiency, respiratory congestion, rhabdomyolysis, sinus bradycardia, skin discoloration, skin lesion, skin photosensitivity, sneezing, splenomegaly, Stevens-Johnson syndrome, tachycardia, throat irritation, thrombocytopenia, tinnitus, toxic epidermal necrolysis, transient ischemic attacks, tremor, upper respiratory tract infection, urinary incontinence, urinary urgency, urticaria (including dermographism), vertigo, vomiting, weight gain, weight loss, xerostomia
Warnings/Precautions
Concerns related to adverse effects:
• Cardiovascular death: [US Boxed Warning]: Gout patients with established cardiovascular (CV) disease had a higher rate of CV death when treated with febuxostat compared to patients treated with allopurinol in a CV outcomes study. Febuxostat should only be used in patients who have an inadequate response to a maximally titrated dose of allopurinol, who are intolerant to allopurinol, or when treatment with allopurinol is not advisable. Consider risks and benefits when prescribing or continuing febuxostat therapy. Consider prophylactic low-dose aspirin in patients with a history of CV disease. Monitor patients for signs and symptoms of CV events (White 2018).
- Hepatic failure: Postmarketing cases of hepatic failure (both fatal and nonfatal) have been reported (causal relationship has not been established). In controlled studies, significant hepatic transaminase elevations (>3 x ULN) have occurred (causal relationship not established). Liver function tests should be evaluated at baseline and periodically thereafter; evaluate liver function tests promptly in patients experiencing signs and symptoms of hepatic injury (eg, fatigue, anorexia, right upper quadrant pain, dark urine, jaundice). Interrupt therapy in patients who develop abnormal liver function tests (eg, ALT >3 x ULN); permanently discontinue use if no other explanation for the abnormalities is elucidated and in patients who develop ALT >3 x ULT and serum total bilirubin >2 x ULN. All other patients may be cautiously restarted on febuxostat.
- Hypersensitivity: Hypersensitivity and serious skin reactions (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis, DRESS) have been reported, particularly in patients with prior skin reactions to allopurinol; use with caution if a patient has a history of hypersensitivity reaction to allopurinol.
Disease-related concerns:
- Hepatic impairment: Use with caution in patients with severe hepatic impairment (Child-Pugh class C); has not been studied.
- Secondary hyperuricemia: Use in secondary hyperuricemia has not been studied; avoid use in patients at increased risk of urate formation (eg, malignancy and its treatment; Lesch-Nyhan syndrome).
Dosage forms specific issues:
- Lactose: Contains lactose.
Other warnings/precautions:
- Appropriate use: Administer concurrently with an NSAID or colchicine (up to 6 months) to prevent gout flare, which may occur upon initiation of therapy. Do not use to treat asymptomatic or secondary hyperuricemia.
Monitoring Parameters
Liver function tests at baseline and then periodically, serum uric acid levels (as early as 2 weeks after initiation); signs/symptoms of cardiovascular events; signs/symptoms of hypersensitivity or severe skin reactions
Pregnancy
Pregnancy Considerations
Adverse events were observed in some animal reproduction studies.
Patient Education
What is this drug used for?
- It is used to lower uric acid in the blood in people with gout.
Frequently reported side effects of this drug
- Joint pain
- Nausea
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Severe cerebrovascular disease like change in strength on one side is greater than the other, difficulty speaking or thinking, change in balance, or vision changes.
- Stevens-Johnson syndrome/toxic epidermal necrolysis like red, swollen, blistered, or peeling skin (with or without fever); red or irritated eyes; or sores in mouth, throat, nose, or eyes.
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Chest pain
- Fast heartbeat
- Abnormal heartbeat
- Shortness of breath
- Dizziness
- Passing out
- Headache
- Swollen glands
- Flu-like signs
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.