Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet Extended Release 24 Hour, Oral:
Generic: 2.5 mg, 5 mg, 10 mg
Pharmacology
Mechanism of Action
Inhibits calcium ions from entering the “slow channels” or select voltage-sensitive areas of vascular smooth muscle and myocardium during depolarization, producing a relaxation of coronary vascular smooth muscle and coronary vasodilation; increases myocardial oxygen delivery in patients with vasospastic angina
Pharmacokinetics/Pharmacodynamics
Absorption
100%; absolute: 20% due to first-pass effect
Metabolism
Hepatic; CYP3A4 substrate (major); extensive first-pass effect
Excretion
Urine (70% as metabolites); feces 10%
Onset of Action
Antihypertensive: 2 to 5 hours
Time to Peak
2.5 to 5 hours
Duration of Action
Antihypertensive effect: 24 hours
Half-Life Elimination
Immediate release: 11 to 16 hours
Protein Binding
>99%
Use in Specific Populations
Special Populations: Hepatic Function Impairment
Felodipine clearance is reduced about 60% in patients with hepatic impairment.
Special Populations: Elderly
Plasma concentrations of felodipine increase with advancing age. Clearance of felodipine in elderly hypertensive patients was 45% of that observed in young volunteers. Steady-state mean area under curve in young patients was 39% of that observed in elderly patients.
Use: Labeled Indications
Hypertension: Management of hypertension
Use: Off Label
Chronic stable anginaayes
Data from randomized, double-blind, placebo-controlled trials support the use of felodipine for the management of chronic stable angina, primarily in patients who continue to be symptomatic on an optimal dose of a beta-blocker Dunselman 1997, Emanuelsson 1999, Rønnevik 1995.
Based on the 2012 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guideline for the diagnosis and management of patients with stable ischemic heart disease, a dihydropyridine calcium channel blocker (eg, felodipine) is an effective and recommended agent in the management of chronic stable angina. A beta-blocker is the preferred initial therapy for chronic stable angina; however, a dihydropyridine calcium channel blocker (eg, felodipine) may be added if there are ongoing anginal symptoms or may be used as alternative therapy if there are contraindications or unacceptable adverse effects with beta-blockade.
Contraindications
Hypersensitivity to felodipine or any component of the formulation.
Canadian labeling: Additional contraindications (not in US labeling): Hypersensitivity to other dihydropyridines; women of childbearing potential, in pregnancy, and during lactation.
Dosage and Administration
Dosing: Adult
Chronic stable angina (alternative agent) (off-label use): Note: A beta-blocker is the preferred initial therapy; if there are ongoing symptoms on beta-blocker therapy, a calcium channel blocker (typically a dihydropyridine [eg, felodipine]) may be added; felodipine may be used as an alternative therapy if there are contraindications or unacceptable adverse effects with beta-blockade (ACC/AHA [Fihn 2012]).
Oral: Initial: 5 to 10 mg once daily; if initiated at 5 mg, increase dose to 10 mg once daily as tolerated after 2 to 4 weeks (Dunselman 1997; Emanuelsson 1999; Rønnevik 1995)
Hypertension: Note: For initial treatment in patients with blood pressure ≥20/10 mm Hg above goal, may be used in combination with another appropriate agent (eg, ACE inhibitor, ARB, or thiazide diuretic). For patients <20/10 mm Hg above goal, some experts recommend an initial trial of monotherapy; however, over time, many patients will require combination therapy (ACC/AHA [Whelton 2017]; Mann 2019).
Oral: Initial: 2.5 to 5 mg once daily; titrate every 1 to 2 weeks as needed based on patient response; usual dose range: 2.5 to 10 mg once daily (ACC/AHA [Whelton 2017]; Mann 2019)
Dosing: Geriatric
Hypertension: Oral: Consider lower initial doses (eg, 2.5 mg once daily) and titrate at no less than 2-week intervals to response (Aronow 2011).
Dosing: Pediatric
Hypertension: Limited data available: Children ≥6 years and Adolescents: Oral: Initial: 2.5 mg once daily; may increase as needed at 2-week intervals to a maximum daily dose: 10 mg/day (AAP [Flynn 2017]; NHLBI 2011; Trachtmen 2003)
Administration
Oral: Swallow tablet whole; tablet should not be divided, crushed, or chewed. May be administered without food or with a small meal that is low in fat and carbohydrates.
Dietary Considerations
May be taken with a small meal that is low in fat and carbohydrates.
Storage
Store below 30°C (86°F); protect from light.
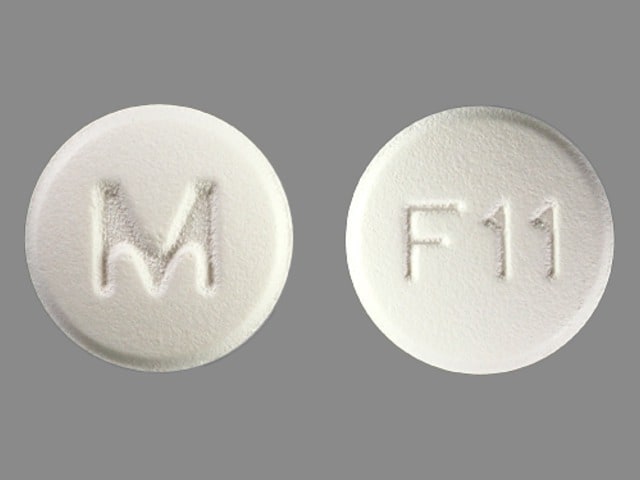
Felodipine Images
Drug Interactions
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Alpha1-Blockers: May enhance the hypotensive effect of Calcium Channel Blockers. Monitor therapy
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Antifungal Agents (Azole Derivatives, Systemic): May enhance the adverse/toxic effect of Calcium Channel Blockers. Specifically, itraconazole may enhance the negative inotropic effects of verapamil or diltiazem. Antifungal Agents (Azole Derivatives, Systemic) may decrease the metabolism of Calcium Channel Blockers. Fluconazole and isavuconazonium likely exert weaker effects than other azoles and are addressed in separate monographs. Management: Concurrent use of felodipine or nisoldipine with itraconazole is specifically contraindicated. Frequent monitoring is warranted with any such combination; calcium channel blocker dose reductions may be required. Exceptions: Fluconazole; Isavuconazonium Sulfate. Consider therapy modification
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Aprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
ARIPiprazole: CYP2D6 Inhibitors (Weak) may increase the serum concentration of ARIPiprazole. Management: Monitor for increased aripiprazole pharmacologic effects. Aripiprazole dose adjustments may or may not be required based on concomitant therapy and/or indication. Consult full interaction monograph for specific recommendations. Monitor therapy
Atosiban: Calcium Channel Blockers may enhance the adverse/toxic effect of Atosiban. Specifically, there may be an increased risk for pulmonary edema and/or dyspnea. Monitor therapy
Barbiturates: May increase the metabolism of Calcium Channel Blockers. Management: Monitor for decreased therapeutic effects of calcium channel blockers with concomitant barbiturate therapy. Calcium channel blocker dose adjustments may be necessary. Nimodipine Canadian labeling contraindicates concomitant use with phenobarbital. Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bitter Orange: May increase the serum concentration of Felodipine. Monitor therapy
Bosentan: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Calcium Channel Blockers (Nondihydropyridine): Calcium Channel Blockers (Dihydropyridine) may enhance the hypotensive effect of Calcium Channel Blockers (Nondihydropyridine). Calcium Channel Blockers (Nondihydropyridine) may increase the serum concentration of Calcium Channel Blockers (Dihydropyridine). Monitor therapy
Calcium Salts: May diminish the therapeutic effect of Calcium Channel Blockers. Monitor therapy
CarBAMazepine: May increase the metabolism of Calcium Channel Blockers (Dihydropyridine). Management: Consider calcium channel blocker (CCB) dose adjustments or alternative therapy in patients receiving concomitant carbamazepine. Nimodipine Canadian labeling contraindicates concurrent use with carbamazepine. Consider therapy modification
Cimetidine: May increase the serum concentration of Calcium Channel Blockers. Management: Consider alternatives to cimetidine. If no suitable alternative exists, monitor for increased effects of calcium channel blockers following cimetidine initiation/dose increase, and decreased effects following cimetidine discontinuation/dose decrease. Consider therapy modification
Clofazimine: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Clopidogrel: Calcium Channel Blockers may diminish the therapeutic effect of Clopidogrel. Monitor therapy
Conivaptan: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
CycloSPORINE (Systemic): Calcium Channel Blockers (Dihydropyridine) may increase the serum concentration of CycloSPORINE (Systemic). CycloSPORINE (Systemic) may increase the serum concentration of Calcium Channel Blockers (Dihydropyridine). Monitor therapy
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May increase the metabolism of CYP3A4 Substrates (High risk with Inducers). Management: Consider an alternative for one of the interacting drugs. Some combinations may be specifically contraindicated. Consult appropriate manufacturer labeling. Consider therapy modification
CYP3A4 Inhibitors (Moderate): May decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CYP3A4 Inhibitors (Strong): May decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Consider therapy modification
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Dapoxetine: May enhance the orthostatic hypotensive effect of Calcium Channel Blockers. Monitor therapy
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
Duvelisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Efavirenz: May decrease the serum concentration of Calcium Channel Blockers. Monitor therapy
Enzalutamide: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Concurrent use of enzalutamide with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP3A4 substrate should be performed with caution and close monitoring. Consider therapy modification
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Erdafitinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fluconazole: May increase the serum concentration of Calcium Channel Blockers. Monitor therapy
Fosaprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fosnetupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fosphenytoin: Calcium Channel Blockers may increase the serum concentration of Fosphenytoin. Management: Monitor for phenytoin toxicity with concomitant use of a calcium channel blocker (CCB) or decreased phenytoin effects with CCB discontinuation. Monitor for decreased CCB therapeutic effects. Nimodipine Canadian labeling contraindicates use with phenytoin. Consider therapy modification
Fusidic Acid (Systemic): May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Grapefruit Juice: May increase the serum concentration of Felodipine. Management: Monitor hemodynamic response to felodipine closely in patients who consume grapefruit juice. Felodipine dose adjustment and/or modification of grapefruit juice ingestion may be needed. Felodipine Canadian labeling recommends avoiding grapefruit juice. Consider therapy modification
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Idelalisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Itraconazole: May increase the serum concentration of Felodipine. Avoid combination
Ivosidenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Ketoconazole (Systemic): May increase the serum concentration of Felodipine. Avoid combination
Larotrectinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Macrolide Antibiotics: May decrease the metabolism of Calcium Channel Blockers. Management: Consider using a noninteracting macrolide. Felodipine Canadian labeling specifically recommends avoiding its use in combination with clarithromycin. Exceptions: Azithromycin (Systemic); Fidaxomicin; Roxithromycin; Spiramycin. Consider therapy modification
Magnesium Salts: Calcium Channel Blockers may enhance the adverse/toxic effect of Magnesium Salts. Magnesium Salts may enhance the hypotensive effect of Calcium Channel Blockers. Monitor therapy
Melatonin: May diminish the antihypertensive effect of Calcium Channel Blockers (Dihydropyridine). Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
MiFEPRIStone: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Minimize doses of CYP3A4 substrates, and monitor for increased concentrations/toxicity, during and 2 weeks following treatment with mifepristone. Avoid cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, and tacrolimus. Consider therapy modification
Mitotane: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Doses of CYP3A4 substrates may need to be adjusted substantially when used in patients being treated with mitotane. Consider therapy modification
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Netupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Neuromuscular-Blocking Agents (Nondepolarizing): Calcium Channel Blockers may enhance the neuromuscular-blocking effect of Neuromuscular-Blocking Agents (Nondepolarizing). Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Palbociclib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Perhexiline: CYP2D6 Inhibitors (Weak) may increase the serum concentration of Perhexiline. Monitor therapy
Phenytoin: Calcium Channel Blockers may increase the serum concentration of Phenytoin. Phenytoin may decrease the serum concentration of Calcium Channel Blockers. Management: Avoid use of nimodipine or nifedipine with phenytoin. Monitor for phenytoin toxicity and/or decreased calcium channel blocker effects with any concurrent use. Consider therapy modification
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Rifamycin Derivatives: May decrease the serum concentration of Calcium Channel Blockers. This primarily affects oral forms of calcium channel blockers. Management: The labeling for some US and Canadian calcium channel blockers contraindicate use with rifampin, however recommendations vary. Consult appropriate labeling. Consider therapy modification
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Simeprevir: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Sincalide: Drugs that Affect Gallbladder Function may diminish the therapeutic effect of Sincalide. Management: Consider discontinuing drugs that may affect gallbladder motility prior to the use of sincalide to stimulate gallbladder contraction. Consider therapy modification
Stiripentol: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Use of stiripentol with CYP3A4 substrates that are considered to have a narrow therapeutic index should be avoided due to the increased risk for adverse effects and toxicity. Any CYP3A4 substrate used with stiripentol requires closer monitoring. Consider therapy modification
Tacrolimus (Systemic): Calcium Channel Blockers (Dihydropyridine) may increase the serum concentration of Tacrolimus (Systemic). Monitor therapy
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
May lead to false-negative aldosterone/renin ratio (ARR) (Funder 2016).
Adverse Reactions
>10%:
Cardiovascular: Peripheral edema (2% to 17%)
Central nervous system: Headache (11% to 15%)
1% to 10%: Cardiovascular: Flushing (4% to 7%), tachycardia (≤3%)
<1%, postmarketing, and/or case reports: Abdominal pain, acid regurgitation, anemia, angina pectoris, angioedema, anxiety disorder, arm pain, arthralgia, back pain, bronchitis, bruise, cardiac arrhythmia, cardiac failure, cerebrovascular accident, chest pain, constipation, decreased libido, depression, diarrhea, dizziness, drowsiness, dyspnea, dysuria, epistaxis, erythema, extrasystoles, facial edema, flatulence, flu-like symptoms, flushing, foot pain, gingival hyperplasia, gynecomastia, hip pain, hypersensitivity angiitis, hypotension, impotence, influenza, insomnia, irritability, knee pain, leg pain, muscle cramps, myalgia, myocardial infarction, nausea, nervousness, palpitations, paresthesia, pharyngitis, polyuria, respiratory tract infection, sinusitis, syncope, urinary frequency, urinary urgency, urticaria, visual disturbance, vomiting, xerostomia
Warnings/Precautions
Concerns related to adverse effects:
- Angina/MI: Increased angina and/or MI has occurred with initiation or dosage titration of dihydropyridine calcium channel blockers; reflex tachycardia may occur resulting in angina and/or MI in patients with obstructive coronary disease especially in the absence of concurrent beta-blockade.
- Hypotension/syncope: Symptomatic hypotension with or without syncope can rarely occur; blood pressure must be lowered at a rate appropriate for the patient's clinical condition.
- Peripheral edema: The most common side effect is peripheral edema (dose dependent); occurs within 2 to 3 weeks of starting therapy.
Disease-related concerns:
- Aortic stenosis: Use with extreme caution in patients with severe aortic stenosis; may reduce coronary perfusion resulting in ischemia.
- Heart failure: The ACC/AHA heart failure guidelines recommend to avoid use in patients with heart failure (HF) due to lack of benefit and/or worse outcomes with calcium channel blockers in general (ACC/AHA [Yancy 2013]).
- Hepatic impairment: Use with caution in patients with hepatic impairment; may require lower starting dose.
- Hypertrophic cardiomyopathy (HCM) with outflow tract obstruction: Use with caution in patients with HCM and outflow tract obstruction since reduction in afterload may worsen symptoms associated with this condition.
Special populations:
- Elderly: Initiate at a lower dose in the elderly.
Dosage forms specific issues:
- Lactose: May contain lactose; if necessary, consider alternative agents in patients intolerant of lactose.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Monitoring Parameters
Blood pressure, heart rate
Hypertension: The 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults (ACC/AHA [Whelton 2017]):
Confirmed hypertension and known CVD or 10-year atherosclerotic cardiovascular disease (ASCVD) risk ≥10%: Target blood pressure <130/80 mm Hg is recommended.
Confirmed hypertension without markers of increased ASCVD risk: Target blood pressure <130/80 mm Hg may be reasonable.
Diabetes and hypertension: The American Diabetes Association (ADA) guidelines (ADA 2019):
Patients 18 to 65 years of age, without ASCVD, and 10-year ASCVD risk <15%: Target blood pressure <140/90 mm Hg is recommended.
Patients 18 to 65 years of age and known ASCVD or 10-year ASCVD risk >15%: Target blood pressure <130/80 mm Hg may be appropriate if it can be safely attained.
Patients >65 years of age (healthy or complex/intermediate health): Target blood pressure <140/90 mm Hg is recommended.
Patients >65 years of age (very complex/poor health): Target blood pressure <150/90 mm Hg is recommended.
Pregnancy
Pregnancy Risk Factor
C
Pregnancy Considerations
Adverse events were observed in animal reproduction studies.
Chronic maternal hypertension may increase the risk of birth defects, low birth weight, preterm delivery, stillbirth, and neonatal death. Actual fetal/neonatal risks may be related to duration and severity of maternal hypertension. Untreated hypertension may also increase the risks of adverse maternal outcomes, including gestational diabetes, myocardial infarction, preeclampsia, stroke, and delivery complications (ACOG 203 2019).
Calcium channel blockers may be used to treat hypertension in pregnant women; however, agents other than felodipine are more commonly used (ACOG 203 2019; ESC [Regitz-Zagrosek 2018]). Females with preexisting hypertension may continue their medication during pregnancy unless contraindications exist (ESC [Regitz-Zagrosek 2018]).
Patient Education
What is this drug used for?
- It is used to treat high blood pressure.
Frequently reported side effects of this drug
- Headache
- Gingival changes
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Fast heartbeat
- Severe dizziness
- Passing out
- Chest pain
- Shortness of breath
- Excessive weight gain
- Swelling of arms or legs
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.