Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Kit, Injection, as acetate:
Generic: 1 mg/0.2 mL
Kit, Intramuscular, as acetate:
Lupron Depot (1-Month): 7.5 mg [latex free; contains polysorbate 80]
Lupron Depot (6-Month): 45 mg [latex free; contains polysorbate 80]
Kit, Intramuscular, as acetate [preservative free]:
Lupron Depot (1-Month): 3.75 mg [latex free; contains polysorbate 80]
Lupron Depot (3-Month): 11.25 mg, 22.5 mg [latex free; contains polysorbate 80]
Lupron Depot (4-Month): 30 mg [latex free; contains polysorbate 80]
Lupron Depot-Ped (1-Month): 7.5 mg, 11.25 mg, 15 mg [latex free; contains polysorbate 80]
Lupron Depot-Ped (3-Month): 30 mg (Ped), 11.25 mg (Ped) [latex free; contains polysorbate 80]
Kit, Subcutaneous, as acetate:
Eligard: 7.5 mg, 22.5 mg, 30 mg, 45 mg
Pharmacology
Mechanism of Action
Leuprolide, is an agonist of gonadotropin releasing hormone (GnRH) receptors. Acting as a potent inhibitor of gonadotropin secretion, leuprolide produces an initial increase in luteinizing hormone (LH) and follicle stimulating hormone (FSH), which leads to a transient increase (5 to 12 days [Cook 2000]) in testosterone and dihydrotestosterone (in males) and estrone and estradione (in premenopausal females). Continuous leuprolide administration then results in suppression of ovarian and testicular steroidogenesis due to decreased levels of LH and FSH with subsequent decrease in testosterone (male) and estrogen (female) levels. In males, testosterone levels are reduced to below castrate levels. Leuprolide may also have a direct inhibitory effect on the testes, and act by a different mechanism not directly related to reduction in serum testosterone.
Pharmacokinetics/Pharmacodynamics
Distribution
Males: Vd: 27 L
Metabolism
Major metabolite, pentapeptide (M-1)
Excretion
Urine (<5% as parent and major metabolite)
Onset of Action
Onset of action: Following transient increase, testosterone suppression occurs in ~2 to 4 weeks of continued therapy
Onset of therapeutic suppression for precocious puberty: Leuprolide: 2 to 4 weeks; Leuprolide depot: 1 month
Half-Life Elimination
~3 hours
Protein Binding
43% to 49%
Use: Labeled Indications
Central precocious puberty: Treatment of children with central precocious puberty (CPP). CPP is defined as early onset of secondary sexual characteristics (usually <8 years of age in girls and <9 years of age in boys) associated with pubertal pituitary gonadotropin activation; may have a significantly advanced bone age resulting in diminished adult height.
Limitations of use: Prior to treatment initiation, confirm clinical diagnosis of CPP with blood concentrations of luteinizing hormone (LH) (basal or stimulated with a gonadotropin-releasing hormone [GnRH] analog), sex steroids, and bone age assessment (versus chronological age). Baseline evaluations should include height and weight measurements, diagnostic brain imaging (to rule out intracranial tumor), pelvic/testicular/adrenal ultrasound (to rule out steroid-secreting tumors), human chorionic gonadotropin levels (to rule out a chorionic gonadotropin-secreting tumor), and adrenal steroid measurements (to exclude congenital adrenal hyperplasia).
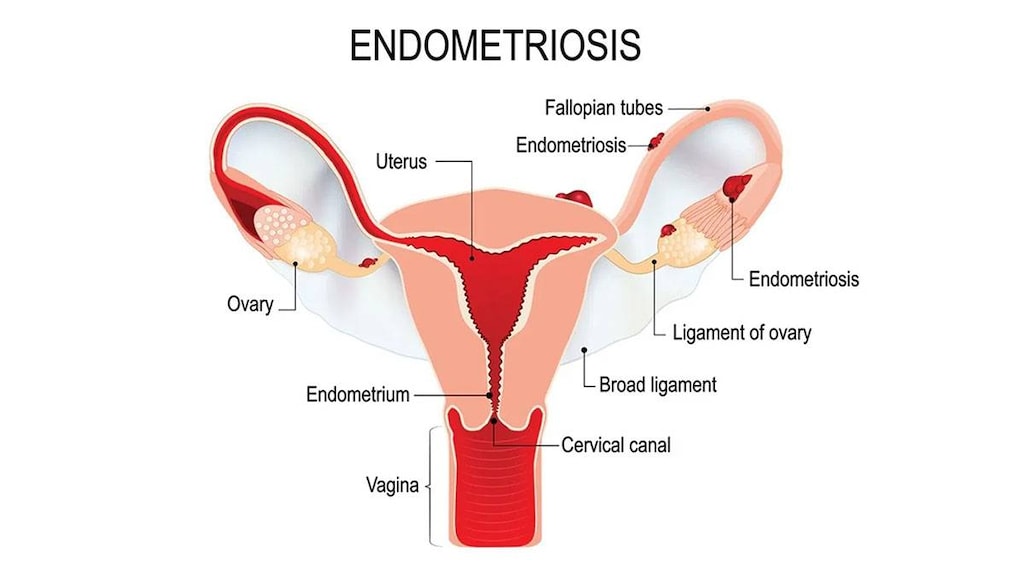
Endometriosis: Management of endometriosis, including pain relief and reduction of endometriotic lesions. Initial management of endometriosis and symptom recurrence (in combination with norethindrone acetate as add-back therapy).
Limitations of use: Initial treatment (maximum 6 months) may be as monotherapy or combination therapy with norethindrone acetate (used as add-back therapy to reduce the loss of bone mineral density). A single retreatment course (maximum 6 months) of leuprolide in combination with norethindrone acetate may be considered if symptoms recur. Monotherapy is not recommended for retreatment. Total duration of therapy should not exceed 12 months.
Prostate cancer, advanced: Palliative treatment of advanced prostate cancer
Uterine leiomyomata (fibroids): Treatment (preoperative) of anemia caused by uterine leiomyomata (fibroids).
Limitations of use: For use in combination with supplemental iron in females with inadequate response to iron alone.
Use: Off Label
Breast cancer, premenopausal ovarian suppressionayes
Data from a large randomized phase III trial, as well as from a smaller randomized study, support the use of leuprolide in premenopausal patients for ovarian suppression in the management of breast cancer Boccardo 1999, Schmid 2007.
Guidelines from the American Society of Clinical Oncology (ASCO) for Endocrine Therapy in Hormone Receptor-Positive Metastatic Breast Cancer recommend that premenopausal women with ER-positive metastatic breast cancer start ovarian suppression, preferably in combination with hormonal therapy. While premenopausal patients without prior hormone therapy exposure can be treated with tamoxifen, or ovarian suppression, or ablation alone, combination therapy is preferred. In metastatic breast cancer, ovarian suppression with GnRH agonists or ablation with oophorectomy appear to achieve similar results.
The ASCO Guideline Update on Ovarian Suppression for Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer recommends that premenopausal women with higher-risk disease receive ovarian suppression (in addition to adjuvant endocrine therapy) although lower-risk patients should not; premenopausal women with stage II or stage III breast cancers who would ordinarily be advised to receive adjuvant chemotherapy should also receive ovarian suppression (in addition to endocrine therapy). Additionally, women with stage I or II breast cancers at higher risk of recurrence who might consider chemotherapy may be offered ovarian suppression (in addition to endocrine therapy).
Hormone therapy for transgender females (male-to-female)yes
Based on the Endocrine Society guidelines for the endocrine treatment of gender dysphoric/gender incongruent persons, gonadotropin-releasing hormone agonists like leuprolide are effective when used in combination with estrogen supplementation for suppressing gonadotropin secretion, thereby decreasing serum testosterone levels into the normal range for females. The result is decreased hair growth, muscle mass, sexual desire, sperm production, spontaneous erections, and testicular volume, as well as breast growth, male sexual dysfunction, redistribution of body fat, and skin and voice changes Dittrich 2005, ES [Hembree 2017].
Paraphiliac
Clinical experience based on multiple clinical trials suggest that leuprolide may be useful in the treatment of paraphilia Guay 2009, Reilly 2000. Additional trials may be necessary to further define the role of leuprolide in this condition.
Contraindications
Hypersensitivity to leuprolide, GnRH, GnRH-agonist analogs, or any component of the formulation; women who are or may become pregnant; breastfeeding (Lupron Depot 3.75 mg [monthly] and Lupron Depot 11.25 mg [3-month]); undiagnosed abnormal vaginal bleeding (Lupron Depot 3.75 mg [monthly] and Lupron Depot 11.25 mg [3-month]).
Lupron Depot 22.5 mg, 30 mg, and 45 mg and Eligard (all strengths) are also not indicated for use in women
Dosage and Administration
Dosing: Adult
Prostate cancer, advanced: Note: Treatment is usually continued after development of metastatic (castration-resistant) disease.
IM:
Lupron Depot 7.5 mg (monthly): 7.5 mg every month or
Lupron Depot 22.5 mg (3 month): 22.5 mg every 12 weeks or
Lupron Depot 30 mg (4 month): 30 mg every 16 weeks or
Lupron Depot 45 mg (6 month): 45 mg every 24 weeks
SubQ:
Eligard: 7.5 mg monthly or 22.5 mg every 3 months or 30 mg every 4 months or 45 mg every 6 months
Leuprolide acetate 5 mg/mL solution: 1 mg daily
Endometriosis: IM: Note: Total duration of therapy (initial plus re-treatment for symptom recurrence) should not exceed 12 months.
Initial therapy: May be used as monotherapy or in combination with norethindrone acetate.
Lupron Depot: 3.75 mg every month for up to 6 months or
Lupron Depot-3 month: 11.25 mg every 3 months for 1 to 2 doses (maximum 6 months)
Symptom recurrence: Administer in combination with norethindrone acetate.
Lupron Depot: 3.75 mg every month for up to 6 months or
Lupron Depot-3 month: 11.25 mg every 3 months for 1 to 2 doses (maximum 6 months)
Uterine leiomyomata (fibroids): IM (in combination with iron):
Lupron Depot: 3.75 mg every month for up to 3 months or
Lupron Depot-3 month: 11.25 mg as a single injection
Breast cancer, premenopausal ovarian supression (off-label use): IM:
Lupron Depot: 3.75 mg every 28 days for up to 24 months (Boccardo 1999) or
Lupron Depot-3 month: 11.25 mg every 3 months for up to 24 months (Boccardo 1999; Schmid 2007)
Hormone therapy for transgender females (male-to-female) (adjunct) (off-label use): Depot IM or SubQ: 3.75 mg monthly or 11.25 mg every 3 months in combination with other appropriate agents (ES [Hembree 2017]; Gava 2016). Lupron Depot should be administered IM and Eligard should be administered SubQ.
Paraphilia (off-label use; Guay 2009; Reilly 2000): Males: IM:
Note: Additional trials may be necessary to further define the role of leuprolide in this condition. May cause an initial increase in androgen concentrations which may be treated with an antiandrogen (eg, flutamide, cyproterone) for 1 to 2 months (Guay 2009). Avoid use in patients with osteoporosis or active pituitary pathology.
SubQ: Test dose: 1 mg (observe for hypersensitivity)
Depot IM: 3.75 to 7.5 mg monthly
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Central precocious puberty (CPP): Note: Prior to initiation of therapy, diagnostic confirmation is necessary and baseline measurements/levels of luteinizing hormone (LH) (basal or stimulated with a gonadotropin-releasing hormone [GnRH] analog), sex steroids, bone age assessment (versus chronological age), and growth parameters (height and weight measurements).
Children ≥2 years: Note: Consider discontinuing leuprolide therapy at the appropriate time for the onset of puberty
Depot Formulations (IM):
Lupron Depot-Ped (monthly formulation):
Weight-directed dosing: Initial: IM: 0.2 to 0.3 mg/kg/dose every 4 weeks; titrate to response (Carel 2009)
Weight-band, fixed dosing:
≤25 kg: Initial: IM: 7.5 mg every 4 weeks; titrate dose in 3.75 mg increments every 4 weeks until clinical or laboratory tests indicate adequate suppression
>25 to 37.5 kg: Initial: IM: 11.25 mg every 4 weeks; titrate dose in 3.75 mg increments every 4 weeks until clinical or laboratory tests indicate adequate suppression
>37.5 kg: Initial: IM: 15 mg every 4 weeks; titrate dose in 3.75 mg increments every 4 weeks until clinical or laboratory tests indicate adequate suppression
Lupron Depot-Ped (3-month formulation): 11.25 mg or 30 mg every 12 weeks
Short-acting formulation (SubQ): Leuprolide acetate 5 mg/mL solution: SubQ: Initial: 50 mcg/kg/dose once daily; may titrate dose upward by 10 mcg/kg/day if suppression of ovarian or testicular steroidogenesis is not achieved.
Endometriosis: Post-menarche Adolescents: Limited data available in ages <18 years: Note: Due to ongoing growth and bone maturation in adolescents, initial therapy with leuprolide should be in combination with norethindrone (add-back therapy) and patients should also be supplemented with calcium and vitamin D (Laufer 2008); if retreatment for an additional 6 months is necessary, concomitant norethindrone should be used. Based on experience in adults, retreatment is not recommended for longer than one additional 6-month course.
Lupron Depot (monthly formulation): IM: 3.75 mg every month for up to 6 months
Lupron Depot (3-month formulation): IM: 11.25 mg every 3 months for up to 2 doses (6 months total duration of treatment)
Leuprolide (GnRHa) Stimulation Test (Female): Limited data available: Children ≥2 years: Leuprolide acetate 5 mg/mL solution: SubQ: 20 mcg/kg once; measure LH and FSH at baseline and after administration (usually two spaced measurements ≤120 minutes [eg, 30 and 60 minutes or 60 and 120 minutes]) (Houk 2008; Sathasivam 2010; Sathasivam 2011)
Uterine leiomyomata (fibroids): Adolescents ≥18 years: Note: Use in combination with iron supplementation for concurrent anemia.
Lupron Depot (monthly formulation): IM: 3.75 mg every month for up to 3 months
Lupron Depot (3-month formulation): IM: 11.25 mg as a single injection
Reconstitution
Eligard: Packaged in two syringes; one contains the Atrigel polymer system and the second contains leuprolide acetate powder; follow package instructions for mixing. Administer within 30 minutes of preparation (discard if not used within 30 minutes).
Lupron Depot, Lupron Depot-Ped: Reconstitute only with diluent provided. Administer within 2 hours of preparation (discard if not used within 2 hours).
Administration
Do not use concurrently a fractional dose of the 3-, 4-, or 6-month depot formulation, or a combination of doses of the monthly depot formulation or any depot formulation due to different release characteristics. Do not use a combination of syringes to achieve a particular dose.
IM: Lupron Depot, Lupron Depot-Ped: Administer as a single injection into the gluteal area, anterior thigh, or deltoid. Vary injection site periodically. Administer within 2 hours of preparation.
SubQ:
Eligard: Vary/rotate injection site; choose site with adequate subcutaneous tissue (eg, upper or mid-abdomen, upper buttocks) that does not have excessive pigment, nodules, lesions, or hair. Avoid areas with brawny or fibrous tissues or areas that may be compressed or rubbed (eg, belt or waistband). Administer within 30 minutes of preparation.
Leuprolide acetate 5 mg/mL solution: Vary injection site; if an alternate syringe from the syringe provided is required, insulin syringes should be used
Storage
Eligard: Store at 2°C to 8°C (36°F to 46°F). Allow to reach room temperature prior to using. Once outside the refrigerator, the kit may be stored (in its original packaging) at 15°C to 30°C (59°F to 86°F) for up to 8 weeks prior to mixing and administration. Once mixed, must be administered within 30 minutes (discard if not used within 30 minutes).
Lupron Depot, Lupron Depot-Ped: Store at room temperature of 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Upon reconstitution, the suspension does not contain a preservative and should be used immediately; discard if not used within 2 hours.
Leuprolide acetate 5 mg/mL solution: Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Protect from light and store vial in carton until use. Do not freeze.
Drug Interactions
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Choline C 11: Luteinizing Hormone-Releasing Hormone Analogs may diminish the therapeutic effect of Choline C 11. Monitor therapy
Corifollitropin Alfa: Luteinizing Hormone-Releasing Hormone Analogs may enhance the therapeutic effect of Corifollitropin Alfa. Avoid combination
Haloperidol: QT-prolonging Agents (Indeterminate Risk - Caution) may enhance the QTc-prolonging effect of Haloperidol. Monitor therapy
Indium 111 Capromab Pendetide: Luteinizing Hormone-Releasing Hormone Analogs may diminish the diagnostic effect of Indium 111 Capromab Pendetide. Avoid combination
QT-prolonging Agents (Highest Risk): QT-prolonging Agents (Indeterminate Risk - Caution) may enhance the QTc-prolonging effect of QT-prolonging Agents (Highest Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Test Interactions
Interferes with pituitary gonadotropic and gonadal function tests during and up to 3 months after monthly administration of leuprolide therapy.
Adverse Reactions
Children (percentages based on 1-month and 3-month pediatric formulations combined):
>10%: Local: Pain at injection site (19% to 20%)
1% to 10%:
Cardiovascular: Vasodilation (2%), bradycardia (<2%), hypertension (<2%), peripheral edema (<2%), peripheral vascular disease (<2%), syncope (<2%)
Central nervous system: Headache (2% to 7%), emotional lability (5%), mood changes (5%), pain (3%), depression (<2%), drowsiness (<2%), nervousness (<2%)
Dermatologic: Skin rash (3%), acne vulgaris (≤3%), erythema multiforme (≤3%), seborrhea (≤3%), alopecia (<2%), body odor (<2%), hair disease (<2%), leukoderma (<2%), nail disease (<2%), nonthrombocytopenic purpura (<2%), skin hypertrophy (<2%)
Endocrine & metabolic: Weight gain (≤7%), feminization (<2%), goiter (<2%), growth suppression (<2%), gynecomastia (<2%), hirsutism (<2%), menstrual disease (females: <2%)
Gastrointestinal: Constipation (<2%), dyspepsia (<2%), dysphagia (<2%), gingivitis (<2%), increased appetite (<2%), nausea (<2%), vomiting (<2%)
Genitourinary: Vaginal discharge (females: ≤3%), vaginal hemorrhage (females: ≤3%), vaginitis (females: ≤3%), breast disease (<2%), cervical neoplasm (females: <2%), cervix disease (females: <2%), dysmenorrhea (females: <2%), urinary incontinence (<2%)
Hematologic & oncologic: Increased erythrocyte sedimentation rate (<2%), tumor flare (<2%)
Hypersensitivity: Hypersensitivity reaction (<2%)
Immunologic: Increased ANA titer (<2%)
Infection: Infection (<2%)
Local: Abscess at injection site (≤9%), injection site reaction (≤9%), swelling at injection site (2%)
Neuromuscular & skeletal: Arthralgia (<2%), arthropathy (<2%), hyperkinesia (<2%), myalgia (<2%), myopathy (<2%)
Ophthalmic: Visual disturbance (<2%)
Respiratory: Asthma (<2%), epistaxis (<2%), flu-like symptoms (<2%), pharyngitis (<2%), rhinitis (<2%), sinusitis (<2%)
Miscellaneous: Fever (<2%)
Frequency not defined:
Cardiovascular: Flushing
Central nervous system: Abnormal gait
Dermatologic: Diaphoresis
Endocrine & metabolic: Diabetes mellitus, hot flash
Adults:
>10%:
Cardiovascular: Flushing (≤58%), ECG changes (≤19%), ischemia (≤19%), edema (≤14%), peripheral edema (≤12%)
Central nervous system: Headache (≤65%), migraine (≤65%), pain (8% to 33%), depression (≤31%), emotional lability (≤31%), insomnia (≤31%), sleep disorder (≤31%), fatigue (≤18%), malaise (≤18%), dizziness (≤16%), vertigo (≤16%), lethargy (≤12%), local discomfort (injection site: ≤11%)
Dermatologic: Diaphoresis (≤98%), allergic skin reaction (≤12%)
Endocrine & metabolic: Hot flash (≤98%), increased serum cholesterol (7% to 59%), increased serum triglycerides (5% to 32%), weight loss (13%), weight gain (≤13%), decreased libido (≤11%)
Gastrointestinal: Nausea (≤25%), vomiting (≤25%), gastrointestinal disease (≤16%), constipation (≤14%), diarrhea (≤14%)
Genitourinary: Vaginitis (females: 11% to 28%), testicular atrophy (males: ≤20%), genitourinary complaint (10% to 15%)
Hematologic & oncologic: Decreased hemoglobin (≤44%; grades 3/4: 1%)
Local: Pain at injection site (≤18%), injection site reaction (≤14%), erythema at injection site (2% to 13%), bruising at injection site (3% to 12%)
Neuromuscular & skeletal: Asthenia (≤18%), arthropathy (8% to 16%)
Respiratory: Flu-like symptoms (≤12%), respiratory tract disease (6% to 11%)
1% to 10%:
Cardiovascular: Hypertension (≤8%), angina pectoris (<5%), atrial fibrillation (<5%), bradycardia (<5%), cardiac arrhythmia (<5%), cardiac failure (<5%), deep vein thrombophlebitis (<5%), hypotension (<5%), myocardial infarction (<5%), palpitations (<5%), pulmonary embolism (<5%), syncope (<5%), tachycardia (<5%), varicose veins (<5%), heart murmur (3%), phlebitis (≤2%), thrombosis (≤2%)
Central nervous system: Anxiety (≤8%), nervousness (≤8%), paresthesia (≤8%), memory impairment (≤6%), ostealgia (<2% to 5%), abnormality in thinking (<5%), agitation (<5%), amnesia (<5%), chills (<5%), confusion (<5%), delusions (<5%), dementia (<5%), hypoesthesia (<5%), loss of consciousness (<5%), neuropathy (<5%), numbness (<5%), paralysis (<5%), peripheral neuropathy (<5%), personality disorder (<5%), seizure (<5%), voice disorder (<5%), altered sense of smell (<2%), rigors (<2%)
Dermatologic: Acne vulgaris (≤10%), dermatological reaction (≤10%), injection site pruritus (≤9%), dermatitis (5%), body odor (<5%), cellulitis (<5%), ecchymoses (<5%), hair disease (<5%), hyperpigmentation (<5%), nail disease (<5%), skin lesion (<5%), xeroderma (<5%), alopecia (≤4%), cold and clammy skin (≤4%), night sweats (≤3%), pruritus (≤3%), skin rash (2%)
Endocrine & metabolic: Decreased HDL cholesterol (2% to 10%), increased LDL cholesterol (8%), dehydration (≤8%), gynecomastia (males: ≤7%), breast changes (≤6%), decreased serum albumin (≥5%), decreased serum bicarbonate (≥5%), decreased serum total protein (≥5%), hypercholesterolemia (≥5%), hyperglycemia (≥5%), hyperlipidemia (≥5%), hyperphosphatemia (≥5%), hyperuricemia (≥5%), increased gamma-glutamyl transferase (≥5%), increased lactate dehydrogenase (≥5%), increased prostatic acid phosphatase (males: ≥5%), diabetes mellitus (≤5%), goiter (<5%), hypercalcemia (<5%), hypoglycemia (<5%), increased thirst (<5%), androgen-like effect (females: ≤4%), menstrual disease (females: ≤2%), loss of libido (<2%), pitting edema (≤1%)
Gastrointestinal: Anorexia (≤6%), abdominal distention (<5%), duodenal ulcer (<5%), dysgeusia (<5%), dysphagia (<5%), eructation (<5%), gastrointestinal hemorrhage (<5%), gingival hemorrhage (<5%), gingivitis (<5%), hernia of abdominal cavity (<5%), hiccups (<5%), increased appetite (<5%), intestinal obstruction (<5%), melanosis (<5%), peptic ulcer (<5%), periodontal abscess (<5%), rectal polyp (<5%), xerostomia (<5%), change in appetite (4%), flatulence (≤4%), mucous membrane abnormality (≤4%), dyspepsia (<4%), colitis (≤3%), gastroenteritis (≤3%)
Genitourinary: Decreased testicular size (males: 7%), mastalgia (≤7%), breast tenderness (≤6%), hematuria (≤6%), urinary frequency (≤6%), urinary urgency (≤6%), impotence (males: ≤5%), balanitis (males: <5%), bladder spasm (<5%), breast hypertrophy (<5%), dysuria (<5%), epididymitis (males: <5%), lactation (females: <5%), penile disease (males: <5%), prostatic disease (males: <5%), testicular disease (males: <5%), urinary incontinence (<5%), urinary tract obstruction (<5%), urinary tract infection (3%), nocturia (≤2%), testicular pain (males: ≤2%), difficulty in micturition (<2%), erectile dysfunction (males: <2%), oliguria (<2%), reduction in penile size (males: <2%), urinary retention (<2%)
Hematologic & oncologic: Decreased prostatic acid phosphatase (males: ≥5%), eosinophilia (≥5%), leukopenia (≥5%), prolonged partial thromboplastin time (≥5%), prolonged prothrombin time (≥5%), thrombocythemia (≥5%), anemia (≤5%), bladder carcinoma (<5%), carcinoma (<5%), lymphadenopathy (<5%), lymphedema (<5%), neoplasm (<5%), skin carcinoma (<5%)
Hepatic: Abnormal hepatic function tests (≥5%), increased liver enzymes (≥5%), increased serum alanine aminotransferase (≥5%), increased serum aspartate aminotransferase (≥5%), hepatomegaly (<5%), increased serum transaminases (3%)
Hypersensitivity: Hypersensitivity reaction (<5%)
Infection: Infection (≤5%), abscess (<5%), herpes zoster infection (<5%)
Local: Induration at injection site (3%)
Neuromuscular & skeletal: Neuromuscular disease (≤10%), myalgia (≤8%), ostealgia (5%), lower limb cramp (≤5%), bone disease (temporal bone swelling: <5%), neck pain (<5%), pathological fracture (<5%), arthralgia (1% to 3%), limb pain (≤3%), musculoskeletal pain (≤2%), amyotrophy (<2%), back pain (<2%), tremor (<2%)
Ophthalmic: Amblyopia (<5%), blepharoptosis (<5%), blurred vision (<5%), conjunctivitis (<5%), ophthalmic signs and symptoms (<5%), visual disturbance (<5%), xerophthalmia (<5%)
Otic: Tinnitus (<5%)
Renal: Decreased urine specific gravity (≥5%), increased urine specific gravity (≥5%)
Respiratory: Paranasal sinus congestion (5%), sinusitis (≤5%), asthma (<5%), bronchitis (<5%), emphysema (<5%), epistaxis (<5%), hemoptysis (<5%), hypoxia (<5%), increased bronchial secretions (<5%), pharyngitis (<5%), pleural effusion (<5%), pleural rub (<5%), pneumonia (<5%), pulmonary disease (<5%), pulmonary edema (<5%), pulmonary fibrosis (<5%), rhinitis (<5%), dyspnea (≤2%), sinus headache (≤2%), cough (1%), dyspnea on exertion (≤1%)
Miscellaneous: Abnormal healing (<5%), accidental injury (<5%), cyst (<5%), fever (<5%), inflammation (<5%)
Frequency not defined:
Cardiovascular: Aortic aneurysm (ruptured), auditory hallucination, carotid stenosis, chest tightness, extrasystoles, facial edema
Central nervous system: Anosmia, burning sensation of feet, euphoria, hyperreflexia, hyporeflexia, motor dysfunction
Dermatologic: Dyschromia, facial swelling, hyperkeratosis, pallor, spider telangiectasia
Endocrine & metabolic: Decreased serum potassium, galactorrhea not associated with childbirth (females), hyperkalemia, increased libido, increased serum albumin, increased serum total protein, thyroid nodule
Gastrointestinal: Glossitis, inguinal hernia, occult blood in stools, rectal fistula, rectal irritation
Genitourinary: Blisters on penis (males), increased post-void residual urine volume, penile swelling (males), prostate pain (males), pyuria, uricosuria
Hematologic & oncologic: Bruise, decreased hematocrit, decreased red blood cells, hypoproteinemia, leukocytosis, second primary malignant neoplasm, thrombocytopenia
Hepatic: Hepatitis, increased serum alkaline phosphatase, increased serum bilirubin
Infection: Influenza
Neuromuscular & skeletal: Ankylosing spondylitis, arthritis, bone fracture, knee effusion, muscle cramps, muscle rigidity, muscle spasm, muscle tenderness
Ophthalmic: Eyelid edema, papilledema, retinal vascular disease (perivascular cuffing)
Renal: Increased blood urea nitrogen, increased serum creatinine, nephrolithiasis, pyelonephritis
Respiratory: Abnormal breath sounds (decreased), chronic obstructive pulmonary disease, dry throat, pleuritic chest pain, pulmonary infiltrates, rales, rhonchi, streptococcal pharyngitis, wheezing
Miscellaneous: Fibrosis (pelvic), mass
<1%, postmarketing, and/or case reports: Abdominal pain, abnormal gait, adenoma (pituitary), aggressive behavior, anaphylactoid shock, anaphylaxis, attempted suicide, auditory disturbance, cerebrovascular accident, chest pain, coronary artery disease, decreased appetite, decreased bone mineral density, decreased white blood cell count, deep vein thrombosis, exacerbation of hematuria, fibromyalgia syndrome, gastrointestinal distress, hematoma at injection site, hepatic injury, hepatic insufficiency, hirsutism, hostility, hyperhidrosis, hypertrichosis, increased testosterone level, induration at injection site, interstitial pulmonary disease, intracranial hypertension (Tan 2019), irritability, lower extremity weakness, nodule (throat), obesity, pituitary apoplexy, severe hepatotoxicity, skin photosensitivity, skin ulceration at injection site, sterile abscess at injection site, suicidal ideation, tenosynovitis (symptoms), thromboembolism, tingling of extremities, transient ischemic attacks, urticaria, venous thrombosis, vertebral column fracture, warm sensation at injection site
Warnings/Precautions
Concerns related to adverse effects:
- Abnormal menses: Females treated for precocious puberty may experience menses or spotting during the first 2 months of treatment; notify health care provider if bleeding continues after the second month.
- Allergic reactions: Anaphylactoid reactions and asthma exacerbations have been reported with therapy in patients with histories of asthma, sinusitis, or environmental or drug allergies.
- Cardiovascular effects: Androgen-deprivation therapy (ADT) may increase the risk for cardiovascular disease (Levine 2010). Sudden cardiac death and stroke have been reported in men receiving GnRH agonists. ADT may prolong the QT/QTc interval; consider the benefits of ADT versus the risk for QT prolongation in patients with a history of QTc prolongation, congenital long QT syndrome, heart failure, frequent electrolyte abnormalities, and in patients with medications known to prolong the QT interval, or with preexisting cardiac disease. Consider periodic monitoring of electrocardiograms and electrolytes in at-risk patients.
- Decreased bone density: Has been reported when used for ≥6 months. Use caution in patients with additional risk factors for bone loss (eg, chronic alcohol or tobacco use, strong family history of osteoporosis, and chronic therapy that may decrease BMD such as corticosteroid or anticonvulsant therapy).
- Endometriosis: Due to the physiologic effects of the drug, exacerbation of endometriosis symptoms may occur after the first dose of leuprolide.
- Hyperglycemia: Diabetes and/or worsening of glycemic control have been reported in men receiving GnRH agonists. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) as clinically necessary.
- Pituitary apoplexy: Rare cases of pituitary apoplexy (frequently secondary to pituitary adenoma) have been observed with GnRH agonist administration (onset from 1 hour to usually <2 weeks); may present as sudden headache, vomiting, visual or mental status changes, and infrequently cardiovascular collapse; immediate medical attention required.
- Psychiatric events: Psychiatric events have been described with GnRH agonists, including leuprolide; symptoms of emotional lability, irritability, impatience, anger, and aggression have been reported in postmarketing accounts. Monitor for development or worsening of psychiatric symptoms. Use with caution in patients with a history of psychiatric illness.
- Seizures: Convulsions have been observed in postmarketing reports in patients receiving GnRH agonists, including leuprolide; patients affected included both those with and without a history of cerebrovascular disorders, CNS anomalies or tumors, epilepsy, seizures, and those on concomitant medications which may lower the seizure threshold (eg, bupropion, SSRIs). If seizures occur, manage accordingly.
- Spinal cord compression: Has been reported when used for prostate cancer; closely observe patients for weakness and paresthesias in first few weeks of therapy. Observe patients with metastatic vertebral lesions closely.
- Tumor flare: Transient increases in testosterone (~50% above baseline) can lead to tumor flare, bone pain, hematuria, bladder outlet obstruction and neuropathy in prostate cancer patients during the first few weeks of therapy.
- Urinary tract obstruction: Has been reported when used for prostate cancer; closely observe patients for urinary tract obstruction and hematuria in first few weeks of therapy. Observe patients with urinary obstruction closely.
Disease-related concerns:
- Depression: Use with caution in patients with depression. Depression may occur or worsen during norethindrone acetate therapy; discontinue if serious depression recurs.
- Prostate cancer: Androgen deprivation therapy may increase the risk for cardiovascular disease, diabetes, insulin resistance, obesity, alterations in lipids, and fractures.
- Uterine fibroids: Fibroid symptoms will recur after therapy cessation; treatment is limited to 3 months’ duration.
Special populations:
- Elderly: Not for use in postmenopausal women.
Concomitant drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Dosage form specific issues:
- Benzyl alcohol and derivatives: Some dosage forms may contain benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol with caution in neonates. See manufacturer’s labeling.
- Depot formulations: Vehicle used in injectable (polylactide-co-glycolide microspheres) has rarely been associated with retinal artery occlusion in patients with abnormal arteriovenous anastomosis (eg, patent foramen ovale). Due to different release properties, combinations of dosage forms or fractions of dosage forms should not be interchanged.
- Eligard Atrigel delivery system: The Atrigel delivery system is a nongelatin-based, biodegradable, polymer matrix.
- Polysorbate 80: Some dosage forms may contain polysorbate 80 (also known as Tweens). Hypersensitivity reactions, usually a delayed reaction, have been reported following exposure to pharmaceutical products containing polysorbate 80 in certain individuals (Isaksson 2002; Lucente 2000; Shelley 1995). Thrombocytopenia, ascites, pulmonary deterioration, and renal and hepatic failure have been reported in premature neonates after receiving parenteral products containing polysorbate 80 (Alade 1986; CDC 1984). See manufacturer’s labeling.
Other warnings/precautions:
- Appropriate use: Breast cancer: The American Society of Clinical Oncology (ASCO) Guideline Update on Ovarian Suppression for Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer (Burstein 2016) recommends that premenopausal women with higher-risk disease receive ovarian suppression (in addition to adjuvant endocrine therapy), although lower-risk patients should not; premenopausal women with stage II or stage III breast cancers who would ordinarily be advised to receive adjuvant chemotherapy should also receive ovarian suppression (in addition to endocrine therapy). Additionally, women with stage I or II breast cancers at higher risk of recurrence who might consider chemotherapy may be offered ovarian suppression (in addition to endocrine therapy). Women with stage 1 disease which does not require chemotherapy should receive endocrine therapy, but not ovarian suppression. Likewise, women with node-negative cancers 1 cm or less (T1a, T1b) should receive endocrine therapy, but not ovarian suppression. Guidelines from ASCO for Endocrine Therapy in Hormone Receptor-Positive Metastatic Breast Cancer (Rugo 2016) recommend that premenopausal women with ER-positive metastatic breast cancer start ovarian suppression, preferably in combination with hormonal therapy. While premenopausal patients without prior hormone therapy exposure can be treated with tamoxifen, or ovarian suppression, or ablation alone, combination therapy is preferred. In metastatic breast cancer, ovarian suppression with GnRH agonists or ablation with oophorectomy appear to achieve similar results.
- Appropriate use: Prostate cancer: Guidelines from the American Society of Clinical Oncology (ASCO) for hormonal management of advanced prostate cancer which is androgen-sensitive (Loblaw 2007) recommend either orchiectomy or luteinizing hormone-releasing hormone (LHRH) agonists as initial treatment for androgen deprivation.
Monitoring Parameters
Bone mineral density; development or worsening of psychiatric symptoms
Endometriosis: Pregnancy test (prior to therapy); endometrial-related pain
Paraphilia (off-label use; Reilly 2000): CBC (baseline, monthly for 4 months then every 6 months); serum testosterone (baseline, monthly for 4 months then every 6 months); serum luteinizing hormone (LH) (baseline and every 6 months), follicle-stimulating hormone (FHS) (baseline), serum BUN and creatinine (baseline and every 6 months); bone density (baseline and yearly); ECG (baseline)
Precocious puberty: GnRH testing (blood LH and FSH levels), measurement of height and bone age every 6 to 12 months, testosterone in males and estradiol in females (IM [monthly] and SubQ formulations: 1 to 2 months after initiation of therapy or with dosage change; IM [3 month] formulation: 2 to 3 months after initiation of therapy, month 6, and as clinically indicated thereafter); Tanner staging
Prostatic cancer: LH and FSH levels, serum testosterone (~4 weeks after initiation of therapy), PSA; weakness, paresthesias, and urinary tract obstruction in first few weeks of therapy. Screen for diabetes (blood glucose and HbA1c) and cardiovascular risk prior to initiating and periodically during treatment. Consider periodic monitoring of electrocardiograms and electrolytes.
Transgender hormone therapy: Serum testosterone levels (goal <50 ng/dL) every 3 months during the first year and then annually or biannually; serum LH, FHS, and prolactin levels at baseline and annually; routine cancer and laboratory screening as in non-transgender individuals for all tissues present (ES [Hembree 2017]; Gava 2016).
Pregnancy
Pregnancy Risk Factor
X
Pregnancy Considerations
Use is contraindicated in pregnant females or females who may become pregnant.
Based on the mechanism of action and data from animal reproduction studies, adverse fetal events may occur following maternal use during pregnancy.
Pregnancy must be excluded prior to the start of treatment. Although leuprolide usually inhibits ovulation and stops menstruation, contraception is not ensured and a nonhormonal contraceptive should be used during therapy.
Patient Education
What is this drug used for?
- It is used to treat endometriosis.
- It is used to treat anemia caused by fibroids of the uterus.
- It is used to treat prostate cancer.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Fatigue
- Loss of strength and energy
- Lack of appetite
- Constipation
- Flu-like signs
- Breast pain or soreness
- Testicle changes
- Sexual dysfunction
- Hot flashes
- Sweating
- Enlarged breasts
- Nausea
- Vomiting
- Trouble sleeping
- Injection site irritation
- Joint pain
- Acne
- Muscle pain
- Anxiety
- Weight loss or gain
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Infection
- Severe cerebrovascular disease like change in strength on one side is greater than the other, trouble speaking or thinking, change in balance, or vision changes
- Pituitary apoplexy like sudden headache, vomiting, passing out, mood changes, eye weakness, unable to move eyes, or vision changes.
- High blood sugar like confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit
- Dehydration like dry skin, dry mouth, dry eyes, increased thirst, fast heartbeat, dizziness, fast breathing, or confusion
- Chest pain
- Severe headache
- Severe dizziness
- Passing out
- Vision changes
- Bone pain
- Unable to pass urine
- Change in amount of urine passed
- Severe back pain
- Seizures
- Blood in the urine
- Burning or numbness feeling
- Shortness of breath
- Slow heartbeat
- Fast heartbeat
- Abnormal heartbeat
- Excessive weight gain
- Swelling of arms or legs
- Trouble moving
- Behavioral changes
- Mood changes
- Menstruation
- Vaginal pain, itching, and discharge
- Vaginal irritation
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.