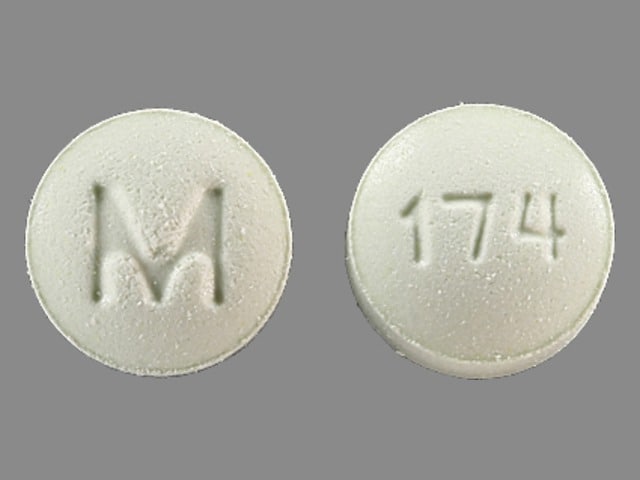
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Generic: 2.5 mg, 5 mg, 10 mg
Pharmacology
Mechanism of Action
Inhibits sodium reabsorption in the distal tubules causing increased excretion of sodium and water, as well as, potassium and hydrogen ions
Pharmacokinetics/Pharmacodynamics
Distribution
Vd: 113 L (Ernst 2009).
Excretion
Urine (80%) (Ernst 2009).
Onset of Action
Diuresis: ~60 minutes.
Duration of Action
≥24 hours.
Half-Life Elimination
8 to 14 hours (Ernst 2009).
Protein Binding
95% (Ernst 2009).
Use in Specific Populations
Special Populations: Renal Function Impairment
Accumulation may occur in severe renal impairment.
Use: Labeled Indications
Edema: Treatment of edema due to heart failure or renal diseases, including the nephrotic syndrome and states of diminished renal function.
Contraindications
Hypersensitivity to metolazone or any component of the formulation; anuria; hepatic coma or precoma.
Documentation of allergenic cross-reactivity for thiazide diuretics is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Dosage and Administration
Dosing: Adult
Edema (heart failure) (adjunctive agent) (off-label dose): Note: A loop diuretic is preferred initial therapy for edema in patients with heart failure with reduced or preserved ejection fraction. It is reasonable to add a thiazide-type diuretic (eg, metolazone) in hospitalized patients with acute decompensated heart failure if IV loop diuretic therapy is inadequate for relieving congestive symptoms (ACCF/AHA [Yancy 2013]).
Oral: Initial: 2.5 mg once daily; maximum daily dose: 20 mg; dosing frequency may be adjusted based on patient-specific diuretic needs (eg, administration every other day or weekly) (ACCF/AHA [Yancy 2013]; HFSA [Lindenfield 2010]).
Edema (renal disease): Oral: Initial: 5 to 20 mg once daily.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Edema, refractory: Limited data available: Infants, Children, and Adolescents: Oral: Usual range: 0.2 to 0.4 mg/kg/day divided every 12 to 24 hours in combination with furosemide; adjust dose to minimal effective dose for maintenance (Arnold 1984; Nelson 1996; Wise 2018); maximum adult daily dose: 20 mg/day. Note: Published efficacy of metolazone in infants and children are limited; underlying disease state, renal function, and concomitant therapies all may affect response (Arnold 1984; Wise 2018). According to the manufacturer, a lower dose of 0.05 to 0.1 mg/kg once daily has also been reported to result in weight loss and increase urine output in some pediatric patients.
Extemporaneously Prepared
A 1 mg/mL oral suspension may be made by with tablets and one of three different vehicles (cherry syrup diluted 1:4 with simple syrup; a 1:1 mixture of Ora-Sweet and Ora-Plus; or a 1:1 mixture of Ora-Sweet SF and Ora-Plus). Crush twelve 10 mg tablets in a mortar and reduce to a fine powder. Add small portions of the chosen vehicle and mix to a uniform paste; mix while adding the vehicle in incremental proportions to almost 120 mL; transfer to a calibrated bottle, rinse mortar with vehicle, and add quantity of vehicle sufficient to make 120 mL. Label "shake well" and "refrigerate". Stable for 60 days.
A 0.25 mg/mL oral suspension may be made with tablets and a 1:1 mixture of methylcellulose 1% and simple syrup. Crush one 2.5 mg tablet in a mortar and reduce to a fine powder. Add small portions of the vehicle and mix to a uniform paste; mix while adding the vehicle in incremental proportions to almost 10 mL; transfer to a calibrated bottle, rinse mortar with vehicle, and add quantity of vehicle sufficient to make 10 mL. Label "shake well" and "refrigerate". Stable for 91 days refrigerated (preferred), 28 days at room temperature in plastic, and 14 days at room temperature in glass.
Nahata, MC, Pai VB, and Hipple TF, Pediatric Drug Formulations, 5th ed, Cincinnati, OH: Harvey Whitney Books Co, 2004.
Administration
Oral: Administer as a single daily dose with or without food. Therapy should be taken early in the day to avoid nocturia.
Dietary Considerations
May require potassium supplementation
Storage
Store at 25°C (77°F); excursions are permitted between 15°C and 30°C (59°F and 86°F). Protect from light.
Metolazone Images
Drug Interactions
Ajmaline: Sulfonamides may enhance the adverse/toxic effect of Ajmaline. Specifically, the risk for cholestasis may be increased. Monitor therapy
Alcohol (Ethyl): May enhance the orthostatic hypotensive effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Allopurinol: Thiazide and Thiazide-Like Diuretics may enhance the potential for allergic or hypersensitivity reactions to Allopurinol. Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Allopurinol. Specifically, Thiazide Diuretics may increase the concentration of Oxypurinol, an active metabolite of Allopurinol. Monitor therapy
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Aminolevulinic Acid (Systemic): Photosensitizing Agents may enhance the photosensitizing effect of Aminolevulinic Acid (Systemic). Avoid combination
Aminolevulinic Acid (Topical): Photosensitizing Agents may enhance the photosensitizing effect of Aminolevulinic Acid (Topical). Monitor therapy
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Angiotensin-Converting Enzyme Inhibitors: Thiazide and Thiazide-Like Diuretics may enhance the hypotensive effect of Angiotensin-Converting Enzyme Inhibitors. Thiazide and Thiazide-Like Diuretics may enhance the nephrotoxic effect of Angiotensin-Converting Enzyme Inhibitors. Monitor therapy
Anticholinergic Agents: May increase the serum concentration of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Antidiabetic Agents: Thiazide and Thiazide-Like Diuretics may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Beta2-Agonists: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Bile Acid Sequestrants: May decrease the absorption of Thiazide and Thiazide-Like Diuretics. The diuretic response is likewise decreased. Consider therapy modification
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Calcium Salts: Thiazide and Thiazide-Like Diuretics may decrease the excretion of Calcium Salts. Continued concomitant use can also result in metabolic alkalosis. Monitor therapy
CarBAMazepine: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of CarBAMazepine. Specifically, there may be an increased risk for hyponatremia. Monitor therapy
Cardiac Glycosides: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Cardiac Glycosides. Specifically, cardiac glycoside toxicity may be enhanced by the hypokalemic and hypomagnesemic effect of thiazide diuretics. Monitor therapy
Corticosteroids (Orally Inhaled): May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Corticosteroids (Systemic): May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Cyclophosphamide: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Cyclophosphamide. Specifically, granulocytopenia may be enhanced. Monitor therapy
Dexketoprofen: May enhance the adverse/toxic effect of Sulfonamides. Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diacerein: May enhance the therapeutic effect of Diuretics. Specifically, the risk for dehydration or hypokalemia may be increased. Monitor therapy
Diazoxide: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Diazoxide. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Dichlorphenamide: Thiazide and Thiazide-Like Diuretics may enhance the hypokalemic effect of Dichlorphenamide. Monitor therapy
Dofetilide: Thiazide and Thiazide-Like Diuretics may enhance the QTc-prolonging effect of Dofetilide. Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Dofetilide. Management: Although hydrochlorothiazide is specifically cited as a contraindication, the risk likely extends to all thiazide and thiazide-like diuretics and may be even greater with chlorthalidone or bendroflumethiazide. Consider alternatives when possible. Consider therapy modification
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
Fexinidazole [INT]: Thiazide and Thiazide-Like Diuretics may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Ipragliflozin: May enhance the adverse/toxic effect of Thiazide and Thiazide-Like Diuretics. Specifically, the risk for intravascular volume depletion may be increased. Monitor therapy
Ivabradine: Thiazide and Thiazide-Like Diuretics may enhance the arrhythmogenic effect of Ivabradine. Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Levosulpiride: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Levosulpiride. Avoid combination
Licorice: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Lithium: Thiazide and Thiazide-Like Diuretics may decrease the excretion of Lithium. Consider therapy modification
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Mecamylamine: Sulfonamides may enhance the adverse/toxic effect of Mecamylamine. Avoid combination
Methenamine: Thiazide and Thiazide-Like Diuretics may diminish the therapeutic effect of Methenamine. Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Multivitamins/Fluoride (with ADE): May enhance the hypercalcemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Multivitamins/Minerals (with ADEK, Folate, Iron): Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Multivitamins/Minerals (with ADEK, Folate, Iron). Monitor therapy
Multivitamins/Minerals (with AE, No Iron): Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Multivitamins/Minerals (with AE, No Iron). Specifically, thiazide diuretics may decrease the excretion of calcium, and continued concomitant use can also result in metabolic alkalosis. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Neuromuscular-Blocking Agents (Nondepolarizing): Thiazide and Thiazide-Like Diuretics may enhance the neuromuscular-blocking effect of Neuromuscular-Blocking Agents (Nondepolarizing). Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: Thiazide and Thiazide-Like Diuretics may enhance the nephrotoxic effect of Nonsteroidal Anti-Inflammatory Agents. Nonsteroidal Anti-Inflammatory Agents may diminish the therapeutic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Opioid Agonists: May enhance the adverse/toxic effect of Diuretics. Opioid Agonists may diminish the therapeutic effect of Diuretics. Monitor therapy
OXcarbazepine: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of OXcarbazepine. Specifically, there may be an increased risk for hyponatremia. Monitor therapy
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Porfimer: Photosensitizing Agents may enhance the photosensitizing effect of Porfimer. Monitor therapy
Promazine: Thiazide and Thiazide-Like Diuretics may enhance the QTc-prolonging effect of Promazine. Avoid combination
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Reboxetine: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Selective Serotonin Reuptake Inhibitors: May enhance the hyponatremic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Sodium Phosphates: Diuretics may enhance the nephrotoxic effect of Sodium Phosphates. Specifically, the risk of acute phosphate nephropathy may be enhanced. Management: Consider avoiding this combination by temporarily suspending treatment with diuretics, or seeking alternatives to oral sodium phosphate bowel preparation. If the combination cannot be avoided, hydrate adequately and monitor fluid and renal status. Consider therapy modification
Topiramate: Thiazide and Thiazide-Like Diuretics may enhance the hypokalemic effect of Topiramate. Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Topiramate. Management: Monitor for increased topiramate levels/adverse effects (e.g., hypokalemia) with initiation/dose increase of a thiazide diuretic. Closely monitor serum potassium concentrations with concomitant therapy. Topiramate dose reductions may be necessary. Consider therapy modification
Toremifene: Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Toremifene. Monitor therapy
Verteporfin: Photosensitizing Agents may enhance the photosensitizing effect of Verteporfin. Monitor therapy
Vitamin D Analogs: Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Vitamin D Analogs. Monitor therapy
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
May lead to false-negative aldosterone/renin ratio (ARR) (Funder 2016)
Adverse Reactions
Frequency not defined.
Cardiovascular: Chest discomfort, chest pain, necrotizing angiitis, orthostatic hypotension, palpitations, syncope, venous thrombosis
Central nervous system: Chills, depression, dizziness, drowsiness, fatigue, headache, neuropathy, paresthesia, restlessness, vertigo
Dermatologic: Pruritus, skin necrosis, skin photosensitivity, skin rash, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria
Endocrine & metabolic: Glycosuria, gout, hypercalcemia, hyperglycemia, hyperuricemia, hypochloremia, hypochloremic alkalosis, hypokalemia, hypomagnesemia, hyponatremia, hypophosphatemia, hypovolemia
Gastrointestinal: Abdominal pain, anorexia, bloating, constipation, diarrhea, epigastric distress, nausea, pancreatitis, vomiting, xerostomia
Genitourinary: Impotence
Hematologic & oncologic: Agranulocytosis, aplastic anemia, hemoconcentration, hypoplastic anemia, leukopenia, petechia, purpura, thrombocytopenia
Hepatic: Cholestatic jaundice, hepatitis
Neuromuscular & skeletal: Arthralgia, muscle cramps, muscle spasm, weakness
Ophthalmic: Transient blurred vision
Renal: Increased blood urea nitrogen
Warnings/Precautions
Concerns related to adverse effects:
- Electrolyte disturbances: Severe hypokalemia and/or hyponatremia can occur rapidly following initial doses. Hypercalcemia, hypochloremic alkalosis, and/or hypomagnesemia can also occur.
- Hypersensitivity: Sensitivity reactions, including angioedema and bronchospasm, may occur.
- Orthostatic hypotension: Orthostatic hypotension may occur. Ethanol, barbiturates, narcotics, or concurrent treatment with other antihypertensive agents may potentiate orthostatic hypotensive effect of metolazone. Instruct patients to avoid these agents during therapy. If taken concurrently, monitor for hypotensive effects.
- Photosensitivity: Photosensitization may occur.
- Renal effects: Azotemia and oliguria may occur.
- Sulfonamide ("sulfa") allergy: The FDA-approved product labeling for many medications containing a sulfonamide chemical group includes a broad contraindication in patients with a prior allergic reaction to sulfonamides. There is a potential for cross-reactivity between members of a specific class (eg, two antibiotic sulfonamides). However, concerns for cross-reactivity have previously extended to all compounds containing the sulfonamide structure (SO2NH2). An expanded understanding of allergic mechanisms indicates cross-reactivity between antibiotic sulfonamides and nonantibiotic sulfonamides may not occur or at the very least this potential is extremely low (Brackett 2004; Johnson 2005; Slatore 2004; Tornero 2004). In particular, mechanisms of cross-reaction due to antibody production (anaphylaxis) are unlikely to occur with nonantibiotic sulfonamides. T-cell-mediated (type IV) reactions (eg, maculopapular rash) are less well understood and it is not possible to completely exclude this potential based on current insights. In cases where prior reactions were severe (Stevens-Johnson syndrome/TEN), some clinicians choose to avoid exposure to these classes.
Disease-related concerns:
- Adrenal insufficiency: Avoid use of diuretics for treatment of elevated blood pressure in patients with primary adrenal insufficiency (Addison disease). Adjustment of glucocorticoid/mineralocorticoid therapy and/or use of other antihypertensive agents is preferred to treat hypertension (Bornstein 2016; Inder 2015).
- Bariatric surgery: Dehydration: Avoid diuretics in the immediate postoperative period after bariatric surgery; electrolyte disturbances and dehydration may occur. Diuretics may be resumed, if indicated, once oral fluid intake goals are met (Ziegler 2009).
- Diabetes: Use with caution in patients with prediabetes or diabetes mellitus; may see a change in glucose control.
- Gout: Hyperuricemia can occur and gout can be precipitated.
- Hepatic impairment: Use with caution in patients with severe hepatic dysfunction.
- Hypokalemia: Use with caution in patients with hypokalemia; correct before initiating therapy.
- Renal impairment: Use caution in severe renal disease. If azotemia and oliguria worsen during treatment in these patients, discontinue therapy.
- Systemic lupus erythematosus (SLE): Can cause SLE exacerbation or activation.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Surgical patients: If given the morning of surgery, metolazone may render the patient volume depleted and blood pressure may be labile during general anesthesia.
Other warnings/precautions:
- Interchangeability: Do not interchange Zaroxolyn with other formulations of metolazone that are not therapeutically equivalent at the same doses (eg, Mykrox, no longer available in the US).
Monitoring Parameters
Serum electrolytes, uric acid, fluid balance, renal function, blood pressure (standing, sitting/supine).
Pregnancy
Pregnancy Considerations
Metolazone crosses the placenta and appears in cord blood.
Hypoglycemia, hypokalemia, hyponatremia, jaundice, and thrombocytopenia are reported as complications to the fetus or newborn following maternal use of thiazide diuretics.
Use to treat edema during normal pregnancies is not appropriate; use may be considered when edema is due to pathologic causes (as in the nonpregnant patient); monitor.
Patient Education
What is this drug used for?
- It is used to treat high blood pressure.
- It is used to get rid of extra fluid.
Frequently reported side effects of this drug
- Constipation
- Diarrhea
- Fatigue
- Headache
- Joint pain
- Lack of appetite
- Nausea
- Vomiting
- Bloating
- Abdominal pain
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Fluid and electrolyte problems like mood changes, confusion, muscle pain or weakness, abnormal heartbeat, severe dizziness, passing out, fast heartbeat, increased thirst, seizures, loss of strength and energy, lack of appetite, unable to pass urine or change in amount of urine passed, dry mouth, dry eyes, or nausea or vomiting.
- Kidney problems like unable to pass urine, blood in the urine, change in amount of urine passed, or weight gain.
- DVT like swelling, warmth, numbness, change in color, or pain in the extremities
- High blood sugar like confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin or eyes
- Pancreatitis like severe abdominal pain, severe back pain, severe nausea, or vomiting
- Severe dizziness
- Passing out
- Burning or numbness feeling
- Chest pain
- Sexual dysfunction
- Bruising
- Bleeding
- Severe loss of strength and energy
- Chills
- Sore throat
- Restlessness
- Depression
- Vision changes
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.