Boxed Warning
Cardiac ischemia after abrupt discontinuation:
Following abrupt discontinuation of therapy with beta adrenergic blockers, exacerbations of angina pectoris and myocardial infarction have occurred.
When discontinuing chronically administered metoprolol/hydrochlorothiazide, particularly in patients with ischemic heart disease, gradually reduce the dose over a period of 1 to 2 weeks and monitor the patient. If angina markedly worsens or acute coronary insufficiency develops, promptly resume therapy, at least temporarily, and take other measures appropriate for the management of unstable angina. Warn patients against interruption or discontinuation of therapy without the health care provider’s advice.
Because coronary artery disease is common and may be unrecognized, avoid abrupt discontinuation of metoprolol/hydrochlorothiazide therapy even in patients treated only for hypertension and in the absence of overt angina pectoris.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Tablet, oral:
Lopressor HCT 50/25: Metoprolol tartrate 50 mg and hydrochlorothiazide 25 mg
Generic: 50/25: Metoprolol tartrate 50 mg and hydrochlorothiazide 25 mg; 100/25: Metoprolol tartrate 100 mg and hydrochlorothiazide 25 mg; 100/50: Metoprolol tartrate 100 mg and hydrochlorothiazide 50 mg
Tablet, extended release, oral:
Dutoprol: 25/12.5: Metoprolol succinate 25 mg [extended release, expressed as mg equivalent to tartrate] and hydrochlorothiazide 12.5 mg
Dutoprol: 50/12.5: Metoprolol succinate 50 mg [extended release, expressed as mg equivalent to tartrate] and hydrochlorothiazide 12.5 mg
Dutoprol 100/12.5: Metoprolol succinate 100 mg [extended release, expressed as mg equivalent to tartrate] and hydrochlorothiazide 12.5 mg [scored]
Generic: 25/12.5: Metoprolol succinate 25 mg [extended release, expressed as mg equivalent to tartrate] and hydrochlorothiazide 12.5 mg [DSC]; 50/12.5: Metoprolol succinate 50 mg [extended release, expressed as mg equivalent to tartrate] and hydrochlorothiazide 12.5 mg [DSC]; 100/12.5: Metoprolol succinate 100 mg [extended release, expressed as mg equivalent to tartrate] and hydrochlorothiazide 12.5 mg [DSC]
Pharmacology
Mechanism of Action
Metoprolol: Selective inhibitor of beta1-adrenergic receptors; competitively blocks beta1-receptors, with little or no effect on beta2-receptors at doses less than 100 mg; does not exhibit any membrane stabilizing or intrinsic sympathomimetic activity.
Hydrochlorothiazide: Inhibits sodium reabsorption in the distal tubules causing increased excretion of sodium and water as well as potassium and hydrogen ions.
Use: Labeled Indications
Hypertension: Management of hypertension.
Contraindications
Hypersensitivity to metoprolol, hydrochlorothiazide, other sulfonamide-derived drugs, other beta-blockers, or any component of the formulation; sinus bradycardia, sick sinus syndrome and greater than first degree heart block (unless permanent pacemaker is in place); cardiogenic shock; overt cardiac failure; anuria
Note: Although the FDA approved product labeling states this medication is contraindicated with other sulfonamide-containing drug classes, the scientific basis of this statement has been challenged. See “Warnings/Precautions” for more detail.
Additional contraindications: Metoprolol tartrate (immediate release)/hydrochlorothiazide: Severe peripheral arterial disease
Dosage and Administration
Dosing: Adult
Hypertension: Oral: Dosage should be determined by titration of the individual agents and the combination product substituted based upon the daily requirements. Note: Hydrochlorothiazide >50 mg/day is not recommended.
Metoprolol tartrate (immediate release)/hydrochlorothiazide: Dosage range: Metoprolol tartrate 100 to 200 mg/hydrochlorothiazide 25 to 50 mg once daily or metoprolol tartrate 50 to 100 mg/hydrochlorothiazide 12.5 to 25 mg twice daily
Concomitant therapy: It is recommended that if an additional antihypertensive agent is required, gradual titration should occur using 1/2 the usual starting dose of the other agent to avoid hypotension.
Metoprolol succinate (extended release)/hydrochlorothiazide: Initial: Metoprolol succinate 25 mg/hydrochlorothiazide 12.5 mg once daily; may titrate once every 2 weeks to maximum of metoprolol succinate 200 mg/hydrochlorothiazide 25 mg once daily
Dosing: Geriatric
Refer to adult dosing.
Administration
To avoid nocturia, doses should be taken early in the day and last dose of multiple doses should be taken no later than 6 pm.
Metoprolol tartrate (immediate release) and hydrochlorothiazide: Administer with or immediately following meals (or as directed).
Metoprolol succinate (extended release) and hydrochlorothiazide: Administer with or without food.
Dietary Considerations
Metoprolol tartrate (immediate release) and hydrochlorothiazide: Take with or immediately following meals (or as directed).
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Protect from moisture.
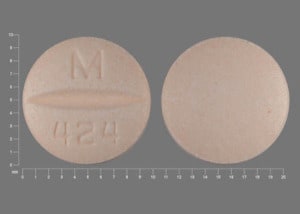
Metoprolol and Hydrochlorothiazide Images
Drug Interactions
Abiraterone Acetate: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Management: Avoid concurrent use of abiraterone with CYP2D6 substrates that have a narrow therapeutic index whenever possible. When concurrent use is not avoidable, monitor patients closely for signs/symptoms of toxicity. Consider therapy modification
Acetylcholinesterase Inhibitors: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Ajmaline: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Ajmaline: Sulfonamides may enhance the adverse/toxic effect of Ajmaline. Specifically, the risk for cholestasis may be increased. Monitor therapy
Alcohol (Ethyl): May enhance the orthostatic hypotensive effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Allopurinol: Thiazide and Thiazide-Like Diuretics may enhance the potential for allergic or hypersensitivity reactions to Allopurinol. Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Allopurinol. Specifically, Thiazide Diuretics may increase the concentration of Oxypurinol, an active metabolite of Allopurinol. Monitor therapy
Alpha1-Blockers: Beta-Blockers may enhance the orthostatic hypotensive effect of Alpha1-Blockers. The risk associated with ophthalmic products is probably less than systemic products. Monitor therapy
Alpha2-Agonists: May enhance the AV-blocking effect of Beta-Blockers. Sinus node dysfunction may also be enhanced. Beta-Blockers may enhance the rebound hypertensive effect of Alpha2-Agonists. This effect can occur when the Alpha2-Agonist is abruptly withdrawn. Management: Closely monitor heart rate during treatment with a beta blocker and clonidine. Withdraw beta blockers several days before clonidine withdrawal when possible, and monitor blood pressure closely. Recommendations for other alpha2-agonists are unavailable. Exceptions: Apraclonidine. Consider therapy modification
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Aminolevulinic Acid (Systemic): Photosensitizing Agents may enhance the photosensitizing effect of Aminolevulinic Acid (Systemic). Avoid combination
Aminolevulinic Acid (Topical): Photosensitizing Agents may enhance the photosensitizing effect of Aminolevulinic Acid (Topical). Monitor therapy
Aminoquinolines (Antimalarial): May decrease the metabolism of Beta-Blockers. Monitor therapy
Amiodarone: May enhance the bradycardic effect of Beta-Blockers. Possibly to the point of cardiac arrest. Amiodarone may increase the serum concentration of Beta-Blockers. Monitor therapy
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Angiotensin-Converting Enzyme Inhibitors: Thiazide and Thiazide-Like Diuretics may enhance the hypotensive effect of Angiotensin-Converting Enzyme Inhibitors. Thiazide and Thiazide-Like Diuretics may enhance the nephrotoxic effect of Angiotensin-Converting Enzyme Inhibitors. Monitor therapy
Anticholinergic Agents: May increase the serum concentration of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Antidiabetic Agents: Thiazide and Thiazide-Like Diuretics may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Antipsychotic Agents (Phenothiazines): May enhance the hypotensive effect of Beta-Blockers. Beta-Blockers may decrease the metabolism of Antipsychotic Agents (Phenothiazines). Antipsychotic Agents (Phenothiazines) may decrease the metabolism of Beta-Blockers. Monitor therapy
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Asunaprevir: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Consider therapy modification
Barbiturates: May decrease the serum concentration of Beta-Blockers. Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benazepril: HydroCHLOROthiazide may enhance the hypotensive effect of Benazepril. HydroCHLOROthiazide may enhance the nephrotoxic effect of Benazepril. Benazepril may decrease the serum concentration of HydroCHLOROthiazide. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Beta2-Agonists: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Beta2-Agonists: Beta-Blockers (Beta1 Selective) may diminish the bronchodilatory effect of Beta2-Agonists. Of particular concern with nonselective beta-blockers or higher doses of the beta1 selective beta-blockers. Monitor therapy
Bile Acid Sequestrants: May decrease the absorption of Thiazide and Thiazide-Like Diuretics. The diuretic response is likewise decreased. Consider therapy modification
Bradycardia-Causing Agents: May enhance the bradycardic effect of other Bradycardia-Causing Agents. Monitor therapy
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Bupivacaine: Beta-Blockers may increase the serum concentration of Bupivacaine. Monitor therapy
Calcium Channel Blockers (Nondihydropyridine): May enhance the hypotensive effect of Beta-Blockers. Bradycardia and signs of heart failure have also been reported. Calcium Channel Blockers (Nondihydropyridine) may increase the serum concentration of Beta-Blockers. Exceptions: Bepridil. Monitor therapy
Calcium Salts: Thiazide and Thiazide-Like Diuretics may decrease the excretion of Calcium Salts. Continued concomitant use can also result in metabolic alkalosis. Monitor therapy
CarBAMazepine: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of CarBAMazepine. Specifically, there may be an increased risk for hyponatremia. Monitor therapy
Cardiac Glycosides: Beta-Blockers may enhance the bradycardic effect of Cardiac Glycosides. Monitor therapy
Cardiac Glycosides: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Cardiac Glycosides. Specifically, cardiac glycoside toxicity may be enhanced by the hypokalemic and hypomagnesemic effect of thiazide diuretics. Monitor therapy
Ceritinib: Bradycardia-Causing Agents may enhance the bradycardic effect of Ceritinib. Management: If this combination cannot be avoided, monitor patients for evidence of symptomatic bradycardia, and closely monitor blood pressure and heart rate during therapy. Exceptions are discussed in separate monographs. Consider therapy modification
Cholinergic Agonists: Beta-Blockers may enhance the adverse/toxic effect of Cholinergic Agonists. Of particular concern are the potential for cardiac conduction abnormalities and bronchoconstriction. Monitor therapy
CloBAZam: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Cobicistat: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Corticosteroids (Orally Inhaled): May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Corticosteroids (Systemic): May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Cyclophosphamide: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Cyclophosphamide. Specifically, granulocytopenia may be enhanced. Monitor therapy
CYP2D6 Inhibitors (Moderate): May increase the serum concentration of Metoprolol. Monitor therapy
CYP2D6 Inhibitors (Strong): May increase the serum concentration of Metoprolol. Monitor therapy
Dacomitinib: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Management: Avoid concurrent use of dacomitinib with CYP2D6 subtrates that have a narrow therapeutic index. Consider therapy modification
Darunavir: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Dexketoprofen: May enhance the adverse/toxic effect of Sulfonamides. Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diacerein: May enhance the therapeutic effect of Diuretics. Specifically, the risk for dehydration or hypokalemia may be increased. Monitor therapy
Diazoxide: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Diazoxide. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Dichlorphenamide: Thiazide and Thiazide-Like Diuretics may enhance the hypokalemic effect of Dichlorphenamide. Monitor therapy
Dipyridamole: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Disopyramide: May enhance the bradycardic effect of Beta-Blockers. Beta-Blockers may enhance the negative inotropic effect of Disopyramide. Monitor therapy
Dofetilide: HydroCHLOROthiazide may enhance the QTc-prolonging effect of Dofetilide. HydroCHLOROthiazide may increase the serum concentration of Dofetilide. Avoid combination
Dronedarone: May enhance the bradycardic effect of Beta-Blockers. Dronedarone may increase the serum concentration of Beta-Blockers. This likely applies only to those agents that are metabolized by CYP2D6. Management: Use lower initial beta-blocker doses; adequate tolerance of the combination, based on ECG findings, should be confirmed prior to any increase in beta-blocker dose. Consider therapy modification
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
EPINEPHrine (Nasal): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Nasal). Monitor therapy
EPINEPHrine (Oral Inhalation): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Oral Inhalation). Monitor therapy
Epinephrine (Racemic): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of Epinephrine (Racemic). Monitor therapy
EPINEPHrine (Systemic): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Systemic). Monitor therapy
Ergot Derivatives: Beta-Blockers may enhance the vasoconstricting effect of Ergot Derivatives. Exceptions: Nicergoline. Consider therapy modification
Fexinidazole [INT]: Thiazide and Thiazide-Like Diuretics may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Fexinidazole [INT]: Bradycardia-Causing Agents may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Fingolimod: Beta-Blockers may enhance the bradycardic effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and beta-blockers if possible. If coadministration is necessary, patients should have overnight continuous ECG monitoring conducted after the first dose of fingolimod. Monitor patients for bradycardia. Consider therapy modification
Floctafenine: May enhance the adverse/toxic effect of Beta-Blockers. Avoid combination
Grass Pollen Allergen Extract (5 Grass Extract): Beta-Blockers may enhance the adverse/toxic effect of Grass Pollen Allergen Extract (5 Grass Extract). More specifically, Beta-Blockers may inhibit the ability to effectively treat severe allergic reactions to Grass Pollen Allergen Extract (5 Grass Extract) with epinephrine. Some other effects of epinephrine may be unaffected or even enhanced (e.g., vasoconstriction) during treatment with Beta-Blockers. Consider therapy modification
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Imatinib: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Insulins: Beta-Blockers may enhance the hypoglycemic effect of Insulins. Monitor therapy
Ipragliflozin: May enhance the adverse/toxic effect of Thiazide and Thiazide-Like Diuretics. Specifically, the risk for intravascular volume depletion may be increased. Monitor therapy
Ivabradine: Thiazide and Thiazide-Like Diuretics may enhance the arrhythmogenic effect of Ivabradine. Monitor therapy
Ivabradine: Bradycardia-Causing Agents may enhance the bradycardic effect of Ivabradine. Monitor therapy
Lacosamide: Bradycardia-Causing Agents may enhance the AV-blocking effect of Lacosamide. Monitor therapy
Lercanidipine: May enhance the hypotensive effect of Metoprolol. Metoprolol may decrease the serum concentration of Lercanidipine. Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Levosulpiride: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of Levosulpiride. Avoid combination
Licorice: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Lidocaine (Systemic): Beta-Blockers may increase the serum concentration of Lidocaine (Systemic). Monitor therapy
Lidocaine (Topical): Beta-Blockers may increase the serum concentration of Lidocaine (Topical). Monitor therapy
Lithium: Thiazide and Thiazide-Like Diuretics may decrease the excretion of Lithium. Consider therapy modification
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Lumefantrine: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Mecamylamine: Sulfonamides may enhance the adverse/toxic effect of Mecamylamine. Avoid combination
Mepivacaine: Beta-Blockers may increase the serum concentration of Mepivacaine. Monitor therapy
Methacholine: Beta-Blockers may enhance the adverse/toxic effect of Methacholine. Monitor therapy
Methenamine: Thiazide and Thiazide-Like Diuretics may diminish the therapeutic effect of Methenamine. Monitor therapy
Methoxyflurane: May enhance the hypotensive effect of Beta-Blockers. Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Midodrine: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Mirabegron: May diminish the antihypertensive effect of Metoprolol. Mirabegron may increase the serum concentration of Metoprolol. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Multivitamins/Fluoride (with ADE): May enhance the hypercalcemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Multivitamins/Minerals (with ADEK, Folate, Iron): Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Multivitamins/Minerals (with ADEK, Folate, Iron). Monitor therapy
Multivitamins/Minerals (with AE, No Iron): Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Multivitamins/Minerals (with AE, No Iron). Specifically, thiazide diuretics may decrease the excretion of calcium, and continued concomitant use can also result in metabolic alkalosis. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Neuromuscular-Blocking Agents (Nondepolarizing): Thiazide and Thiazide-Like Diuretics may enhance the neuromuscular-blocking effect of Neuromuscular-Blocking Agents (Nondepolarizing). Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
NIFEdipine: May enhance the hypotensive effect of Beta-Blockers. NIFEdipine may enhance the negative inotropic effect of Beta-Blockers. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: May diminish the antihypertensive effect of Beta-Blockers. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: Thiazide and Thiazide-Like Diuretics may enhance the nephrotoxic effect of Nonsteroidal Anti-Inflammatory Agents. Nonsteroidal Anti-Inflammatory Agents may diminish the therapeutic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Opioid Agonists: May enhance the adverse/toxic effect of Diuretics. Opioid Agonists may diminish the therapeutic effect of Diuretics. Monitor therapy
Opioids (Anilidopiperidine): May enhance the bradycardic effect of Beta-Blockers. Opioids (Anilidopiperidine) may enhance the hypotensive effect of Beta-Blockers. Monitor therapy
OXcarbazepine: Thiazide and Thiazide-Like Diuretics may enhance the adverse/toxic effect of OXcarbazepine. Specifically, there may be an increased risk for hyponatremia. Monitor therapy
Panobinostat: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Peginterferon Alfa-2b: May decrease the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Peginterferon Alfa-2b may increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Perhexiline: CYP2D6 Substrates (High risk with Inhibitors) may increase the serum concentration of Perhexiline. Perhexiline may increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Porfimer: Photosensitizing Agents may enhance the photosensitizing effect of Porfimer. Monitor therapy
Promazine: Thiazide and Thiazide-Like Diuretics may enhance the QTc-prolonging effect of Promazine. Avoid combination
Propafenone: May increase the serum concentration of Beta-Blockers. Propafenone possesses some independent beta blocking activity. Monitor therapy
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
QuiNINE: May increase the serum concentration of CYP2D6 Substrates (High risk with Inhibitors). Monitor therapy
Reboxetine: May enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Regorafenib: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Reserpine: May enhance the hypotensive effect of Beta-Blockers. Monitor therapy
Rifamycin Derivatives: May decrease the serum concentration of Beta-Blockers. Exceptions: Rifabutin. Monitor therapy
Rivastigmine: May enhance the bradycardic effect of Beta-Blockers. Avoid combination
Ruxolitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Management: Ruxolitinib Canadian product labeling recommends avoiding use with bradycardia-causing agents to the extent possible. Monitor therapy
Selective Serotonin Reuptake Inhibitors: May increase the serum concentration of Beta-Blockers. Exceptions: Citalopram; Escitalopram; FluvoxaMINE. Monitor therapy
Selective Serotonin Reuptake Inhibitors: May enhance the hyponatremic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Siponimod: Bradycardia-Causing Agents may enhance the bradycardic effect of Siponimod. Management: Avoid coadministration of siponimod with drugs that may cause bradycardia. Consider therapy modification
Sodium Phosphates: Diuretics may enhance the nephrotoxic effect of Sodium Phosphates. Specifically, the risk of acute phosphate nephropathy may be enhanced. Management: Consider avoiding this combination by temporarily suspending treatment with diuretics, or seeking alternatives to oral sodium phosphate bowel preparation. If the combination cannot be avoided, hydrate adequately and monitor fluid and renal status. Consider therapy modification
Sulfonylureas: Beta-Blockers may enhance the hypoglycemic effect of Sulfonylureas. Cardioselective beta-blockers (eg, acebutolol, atenolol, metoprolol, and penbutolol) may be safer than nonselective beta-blockers. All beta-blockers appear to mask tachycardia as an initial symptom of hypoglycemia. Ophthalmic beta-blockers are probably associated with lower risk than systemic agents. Monitor therapy
Terlipressin: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Theophylline Derivatives: Beta-Blockers (Beta1 Selective) may diminish the bronchodilatory effect of Theophylline Derivatives. Management: Monitor for reduced theophylline efficacy during concomitant use with any beta-blocker. Beta-1 selective agents are less likely to antagonize theophylline than nonselective agents, but selectivity may be lost at higher doses. Monitor therapy
Tofacitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Topiramate: Thiazide and Thiazide-Like Diuretics may enhance the hypokalemic effect of Topiramate. Thiazide and Thiazide-Like Diuretics may increase the serum concentration of Topiramate. Management: Monitor for increased topiramate levels/adverse effects (e.g., hypokalemia) with initiation/dose increase of a thiazide diuretic. Closely monitor serum potassium concentrations with concomitant therapy. Topiramate dose reductions may be necessary. Consider therapy modification
Toremifene: Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Toremifene. Monitor therapy
Valsartan: HydroCHLOROthiazide may enhance the hypotensive effect of Valsartan. Valsartan may increase the serum concentration of HydroCHLOROthiazide. Monitor therapy
Verteporfin: Photosensitizing Agents may enhance the photosensitizing effect of Verteporfin. Monitor therapy
Vitamin D Analogs: Thiazide and Thiazide-Like Diuretics may enhance the hypercalcemic effect of Vitamin D Analogs. Monitor therapy
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
See individual agents.
Adverse Reactions
Frequency not always defined.
Reactions noted here have been reported with the combination product; see individual drug monographs for additional adverse reactions that may be expected from each agent.
1% to 10%:
Cardiovascular: Bradycardia (6%), edema (1%)
Central nervous system: Drowsiness (10%), headache (10%), lethargy (10%), vertigo (10%), dizziness (3% to 10%), fatigue (3% to 10%), nightmares (1%)
Dermatologic: Diaphoresis (1%)
Endocrine & metabolic: Hypokalemia (<10%), gout (1%)
Gastrointestinal: Anorexia (1%), constipation (1%), diarrhea (1%), nausea (1%), vomiting (1%), xerostomia (1%), pancreatitis
Genitourinary: Impotence (1%)
Hematologic & oncologic: Purpura (1%)
Hepatic: Increased liver enzymes, increased serum bilirubin
Neuromuscular & skeletal: Myalgia (1%)
Ophthalmic: Blurred vision (1%)
Otic: Otalgia (1%), tinnitus (1%)
Respiratory: Flu-like symptoms (10%), nasopharyngitis (≤3%), dyspnea (1%)
Miscellaneous: Decreased exercise tolerance (1%)
Warnings/Precautions
Concerns related to adverse effects:
- Acute renal failure: Patients with severe heart failure or volume depletion may be at increased risk for developing acute renal failure with hydrochlorothiazide
- Anaphylactic reactions: Use caution with history of severe anaphylaxis to allergens; patients taking beta-blockers may become more sensitive to repeated allergen challenges. Treatment of anaphylaxis (eg, epinephrine) in patients taking beta-blockers may be ineffective or promote undesirable effects.
- Bradycardia: Bradycardia, including sinus pause, heart block, and cardiac arrest may occur. Patients with first-degree atrioventricular block, sinus node dysfunction, or conduction disorders (including Wolff-Parkinson-White) may be at increased risk. Monitor heart rate and rhythm; if severe bradycardia occurs, reduce dose or discontinue therapy.
- Electrolyte disturbances: Hypokalemia, hypochloremic alkalosis, hypomagnesemia, and hyponatremia can occur with hydrochlorothiazide. As appropriate, correct electrolyte disturbances prior to initiation.
- Hypersensitivity reactions: Hypersensitivity reactions may occur with hydrochlorothiazide. Risk is increased in patients with a history of allergy or bronchial asthma.
- Ocular effects: Hydrochlorothiazide may cause acute transient myopia and acute angle-closure glaucoma, typically occurring within hours to weeks following initiation; discontinue therapy immediately in patients with acute decreases in visual acuity or ocular pain. Additional treatments may be needed if uncontrolled intraocular pressure persists. Risk factors may include a history of sulfonamide or penicillin allergy.
- Photosensitivity: Photosensitization may occur with hydrochlorothiazide.
- Sulfonamide (“sulfa”) allergy: The FDA-approved product labeling for many medications containing a sulfonamide chemical group includes a broad contraindication in patients with a prior allergic reaction to sulfonamides. There is a potential for cross-reactivity between members of a specific class (eg, two antibiotic sulfonamides). However, concerns for cross-reactivity have previously extended to all compounds containing the sulfonamide structure (SO2NH2). An expanded understanding of allergic mechanisms indicates cross-reactivity between antibiotic sulfonamides and nonantibiotic sulfonamides may not occur or at the very least this potential is extremely low (Brackett 2004; Johnson 2005; Slatore 2004; Tornero 2004). In particular, mechanisms of cross-reaction due to antibody production (anaphylaxis) are unlikely to occur with nonantibiotic sulfonamides. T-cell-mediated (type IV) reactions (eg, maculopapular rash) are less well understood and it is not possible to completely exclude this potential based on current insights. In cases where prior reactions were severe (Stevens-Johnson syndrome/TEN), some clinicians choose to avoid exposure to these classes.
Disease-related concerns:
- Bariatric surgery: Dehydration: Avoid diuretics in the immediate postoperative period after bariatric surgery; electrolyte disturbances and dehydration may occur. Diuretics may be resumed, if indicated, once oral fluid intake goals are met (Ziegler 2009).
- Bronchospastic disease: In general, patients with bronchospastic disease should not receive beta-blockers; however, metoprolol, with B1 selectivity, has been used cautiously with close monitoring. Ensure patient has an inhaled beta2-agonist immediately available.
- Conduction abnormality: Consider preexisting conditions such as sick sinus syndrome before initiating metoprolol. Use is contraindicated in patients with second- or third-degree AV block or marked sinus bradycardia (unless a functioning artificial pacemaker is in place).
- Diabetes: Use with caution in patients with diabetes mellitus; may potentiate hypoglycemia and/or mask signs and symptoms.
- Gout: In certain patients with a history of gout, a familial predisposition to gout, or chronic renal failure, gout can be precipitated by hydrochlorothiazide. This risk may be increased with doses ≥25 mg (Gurwitz 1997).
- Heart failure: Use metoprolol with caution in patients with compensated heart failure and monitor for a worsening of the condition. Use is contraindicated in patients with cardiogenic shock or overt heart failure.
- Hepatic impairment: Use with caution in patients with impaired hepatic function or progressive liver disease. Thiazides may alter fluid and electrolyte balance, which may precipitate hepatic coma.
- Hypercalcemia: Thiazide diuretics may decrease renal calcium excretion; consider avoiding use in patients with hypercalcemia.
- Hypercholesterolemia: Use with caution in patients with moderate or high cholesterol concentrations; increased cholesterol and triglyceride levels have been reported with thiazides.
- Myasthenia gravis: Use metoprolol with caution in patients with myasthenia gravis.
- Parathyroid disease: Thiazide diuretics reduce calcium excretion; pathologic changes in the parathyroid glands with hypercalcemia and hypophosphatemia have been observed with prolonged use; should be discontinued prior to testing for parathyroid function.
- Peripheral vascular disease and Raynaud disease: Metoprolol can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease (PVD) and Raynaud disease. Use with caution and monitor for progression of arterial obstruction.
- Pheochromocytoma (untreated): Adequate alpha-blockade is required prior to use of any beta-blocker.
- Psoriasis: Beta-blocker use has been associated with induction or exacerbation of psoriasis, but cause and effect have not been firmly established.
- Renal impairment: Use with caution in patients with renal impairment; discontinue use with progressive renal impairment. Cumulative effects of hydrochlorothiazide may develop, including azotemia, in patients with impaired renal function. Patients with chronic kidney disease may be at increased risk for developing acute renal failure with hydrochlorothiazide. Use is contraindicated in patients with anuria.
- Systemic lupus erythematosus: Hydrochlorothiazide can cause systemic lupus erythematosus (SLE) exacerbation or activation.
- Thyroid disease: Metoprolol may mask signs of hyperthyroidism (eg, tachycardia). If hyperthyroidism is suspected, carefully manage and monitor; abrupt withdrawal may exacerbate symptoms of hyperthyroidism or precipitate thyroid storm. Alterations in thyroid function tests may be observed.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Elderly: Bradycardia may be observed more frequently in elderly patients (>65 years of age); dosage reductions may be necessary.
Other warnings/precautions:
- Abrupt withdrawal: [US Boxed Warning]: Beta-blocker therapy should not be withdrawn abruptly (particularly in patients with CAD), but gradually tapered over 1 to 2 weeks to avoid acute tachycardia, hypertension, and/or ischemia. Severe exacerbation of angina, ventricular arrhythmias, and myocardial infarction (MI) have been reported following abrupt withdrawal of beta-blocker therapy. Warn patients against interruption or discontinuation of therapy without the health care provider’s advice. Temporary but prompt resumption of therapy may be indicated with markedly worsening of angina or acute coronary insufficiency.
- Appropriate use: Used as a replacement for separate dosing of components or combination when response to single agent is suboptimal; the fixed combination is not indicated for initial treatment of hypertension.
- Major surgery: Although perioperative beta-blocker therapy is recommended prior to elective surgery in selected patients, use of high-dose extended release metoprolol in patients naïve to beta-blocker therapy undergoing noncardiac surgery has been associated with bradycardia, hypotension, stroke, and death. Chronic beta-blocker therapy should not be routinely withdrawn prior to major surgery. If given the morning of surgery, hydrochlorothiazide may render the patient volume depleted and blood pressure may be labile during general anesthesia.
Monitoring Parameters
Blood pressure, heart rate; fluid and electrolyte balance; serum glucose regularly (in patients with diabetes); renal function
Pregnancy
Pregnancy Risk Factor
C
Pregnancy Considerations
Adverse events have been observed in some animal reproduction studies. See individual agents.
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience dizziness, headache, diarrhea, fatigue, flu-like symptoms, or loss of strength and energy. Have patient report immediately to prescriber signs of high blood sugar (confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit), signs of fluid and electrolyte problems (mood changes, confusion, muscle pain or weakness, abnormal heartbeat, severe dizziness, passing out, fast heartbeat, increased thirst, seizures, loss of strength and energy, lack of appetite, unable to pass urine or change in amount of urine passed, dry mouth, dry eyes, or nausea or vomiting), signs of lupus (rash on the cheeks or other body parts, sunburn easy, muscle or joint pain, chest pain or shortness of breath, or swelling in the arms or legs), signs of kidney problems (unable to pass urine, blood in the urine,change in amount of urine passed, or weight gain, chest pain, slow heartbeat, depression, confusion, trouble with memory, shortness of breath, excessive weight gain, swelling of arms or legs, vision changes, or eye pain (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.