Boxed Warning
Progressive multifocal leukoencephalopathy:
Natalizumab increases the risk of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain that usually leads to death or severe disability.
Risk factors for the development of PML include duration of therapy, prior use of immunosuppressants, and presence of anti–JC virus (JCV) antibodies. These factors should be considered in the context of expected benefit when initiating and continuing treatment with natalizumab.
Health care professionals should monitor patients on natalizumab for any new sign or symptom that may be suggestive of PML. Natalizumab dosing should be withheld immediately at the first sign or symptom suggestive of PML. For diagnosis, an evaluation that includes a gadolinium-enhanced magnetic resonance imaging (MRI) scan of the brain and, when indicated, cerebrospinal fluid analysis for JC viral DNA are recommended.
Because of the risk of PML, natalizumab is available only through a restricted program under a risk evaluation and mitigation strategy (REMS) called the TOUCH prescribing program.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Concentrate, Intravenous [preservative free]:
Tysabri: 300 mg/15 mL (15 mL) [contains polysorbate 80]
Pharmacology
Mechanism of Action
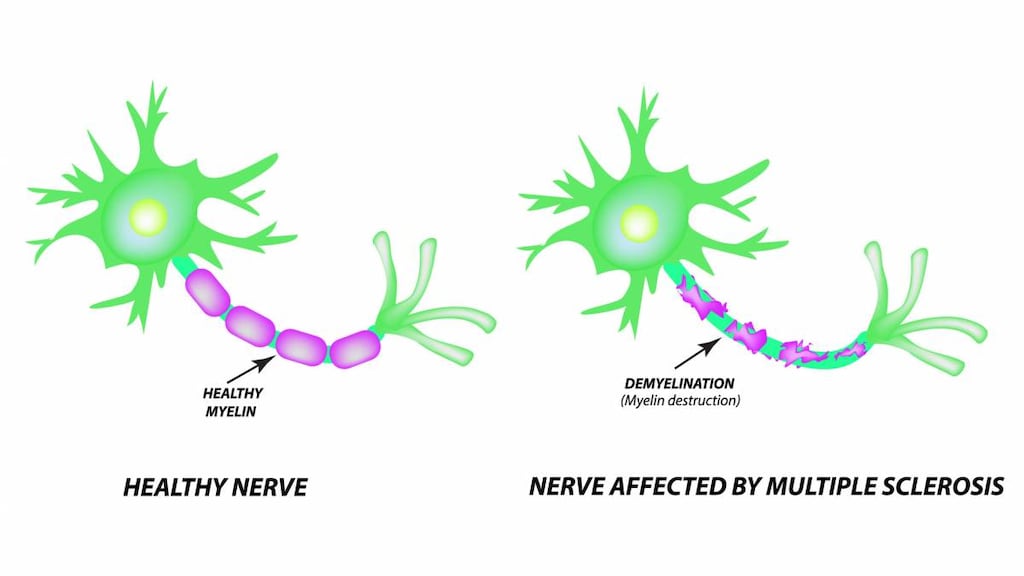
Natalizumab is a monoclonal antibody against the alpha-4 subunit of integrin molecules. These molecules are important to adhesion and migration of cells from the vasculature into inflamed tissue. Natalizumab blocks integrin association with vascular receptors, limiting adhesion and transmigration of leukocytes. Efficacy in specific disorders may be related to reduction in specific inflammatory cell populations in target tissues. In multiple sclerosis, efficacy may be related to blockade of T-lymphocyte migration into the central nervous system; treatment results in a decreased frequency of relapse. In Crohn disease, natalizumab decreases inflammation by binding to alpha-4 integrin, blocking adhesion and migration of leukocytes in the gut.
Pharmacokinetics/Pharmacodynamics
Distribution
Crohn disease: 2.4 to 8 L; Multiple sclerosis: 3.8 to 7.6 L
Half-Life Elimination
Crohn disease: 3 to 17 days; Multiple sclerosis: 7 to 15 days
Use in Specific Populations
Special Populations Note
Antibodies: The presence of persistent anti-natalizumab antibodies increases natalizumab clearance approximately threefold.
Body weight: A less-than-proportional increase in clearance occurs as body weight increases, such that a 43% increase in body weight produces a 32% increase in clearance.
Use: Labeled Indications
Crohn disease: Inducing and maintaining clinical response and remission in adults with moderately to severely active Crohn disease with evidence of inflammation who have had an inadequate response to, or are unable to tolerate, conventional Crohn disease therapies and inhibitors of tumor necrosis factor-alpha (TNF-alpha).
Multiple sclerosis, relapsing: As monotherapy for the treatment of patients with relapsing forms of multiple sclerosis, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease. Natalizumab increases the risk of progressive multifocal leukoencephalopathy. When initiating and continuing treatment with natalizumab, consider whether the expected benefit of natalizumab is sufficient to offset this risk.
Canada labeling: Treatment of relapsing forms of multiple sclerosis in patients who have had an inadequate response to, or are unable to tolerate, other therapies for multiple sclerosis.
Contraindications
Hypersensitivity to natalizumab or any component of the formulation; current or history of progressive multifocal leukoencephalopathy (PML)
Canada labeling: Additional contraindications (not in US labeling): Immunocompromised patients as a result of immunosuppressant or antineoplastic therapy, or immunodeficiencies (eg, HIV, leukemia, lymphoma)
Dosage and Administration
Dosing: Adult
Multiple sclerosis, relapsing: IV: 300 mg infused over 1 hour every 4 weeks.
Crohn disease: IV: 300 mg infused over 1 hour every 4 weeks; discontinue if therapeutic benefit is not observed within initial 12 weeks of therapy.
Concomitant use with corticosteroids: For patients who begin treatment while on chronic oral corticosteroids, begin tapering oral steroids when the onset of natalizumab therapeutic benefit is observed; discontinue use if patient cannot be tapered off of oral corticosteroids within 6 months of therapy initiation. If additional concomitant corticosteroids are required and exceed 3 months/year (in addition to initial corticosteroid taper), consider discontinuing therapy.
Concomitant use with immunosuppressants (eg, azathioprine, cyclosporine, 6-mercaptopurine, or methotrexate) or inhibitors of TNF-alpha: Avoid concomitant use.
Dosing: Geriatric
Refer to adult dosing.
Reconstitution
Dilute natalizumab 300 mg in NS 100 mL to a final concentration of 2.6 mg/mL. Gently invert to mix; do not shake.
Administration
If stored under refrigeration, allow solution to warm to room temperature prior to administration. Diluted solution should be infused over 1 hour; do not administer by IV bolus or push. Patients should be closely monitored for signs and symptoms of hypersensitivity during the infusion and for at least 1 hour after the infusion is complete. The infusion should be discontinued if a reaction occurs, and treatment of the reaction should be instituted. Following infusion, flush line with NS.
Storage
Store concentrated solution under refrigeration between 2°C to 8°C (36°F to 46°F); do not freeze. Protect from light. Do not shake. Following dilution, may be used immediately or refrigerated between 2°C to 8°C (36°F to 46°F) for use within 8 hours; allow diluted solution to warm to room temperature prior to infusion.
Drug Interactions
BCG (Intravesical): Immunosuppressants may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Cladribine: May enhance the immunosuppressive effect of Immunosuppressants. Avoid combination
Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Monitor therapy
Denosumab: May enhance the adverse/toxic effect of Immunosuppressants. Specifically, the risk for serious infections may be increased. Monitor therapy
Echinacea: May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification
Immunosuppressants: May enhance the adverse/toxic effect of Natalizumab. Specifically, the risk of concurrent infection may be increased. Exceptions: Cytarabine (Liposomal). Avoid combination
Nivolumab: Immunosuppressants may diminish the therapeutic effect of Nivolumab. Consider therapy modification
Pidotimod: Immunosuppressants may diminish the therapeutic effect of Pidotimod. Monitor therapy
Pimecrolimus: May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Roflumilast: May enhance the immunosuppressive effect of Immunosuppressants. Consider therapy modification
Sipuleucel-T: Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Management: Evaluate patients to see if it is medically appropriate to reduce or discontinue therapy with immunosuppressants prior to initiating sipuleucel-T therapy. Consider therapy modification
Smallpox and Monkeypox Vaccine (Live): Immunosuppressants may diminish the therapeutic effect of Smallpox and Monkeypox Vaccine (Live). Monitor therapy
Tacrolimus (Topical): May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide. Monitor therapy
Trastuzumab: May enhance the neutropenic effect of Immunosuppressants. Monitor therapy
Upadacitinib: Immunosuppressants may enhance the immunosuppressive effect of Upadacitinib. Avoid combination
Vaccines (Inactivated): Immunosuppressants may diminish the therapeutic effect of Vaccines (Inactivated). Management: Vaccine efficacy may be reduced. Complete all age-appropriate vaccinations at least 2 weeks prior to starting an immunosuppressant. If vaccinated during immunosuppressant therapy, revaccinate at least 3 months after immunosuppressant discontinuation. Consider therapy modification
Vaccines (Live): Immunosuppressants may enhance the adverse/toxic effect of Vaccines (Live). Immunosuppressants may diminish the therapeutic effect of Vaccines (Live). Management: Avoid use of live organism vaccines with immunosuppressants; live-attenuated vaccines should not be given for at least 3 months after immunosuppressants. Exceptions: Smallpox and Monkeypox Vaccine (Live). Avoid combination
Vedolizumab: May enhance the adverse/toxic effect of Natalizumab. Avoid combination
Adverse Reactions
>10%:
Central nervous system: Headache (32% to 38%), fatigue (10% to 27%), depression (≤19%)
Dermatologic: Skin rash (6% to 12%)
Gastrointestinal: Nausea (≤17%), gastroenteritis (≤11%), abdominal distress (≤11%)
Genitourinary: Urinary tract infection (3% to 21%)
Infection: Influenza (≤12%)
Neuromuscular & skeletal: Arthralgia (8% to 19%), limb pain (16%), back pain (≤12%)
Respiratory: Upper respiratory tract infection (≤22%), lower respiratory tract infection (≤17%), flu-like symptoms (≤11%)
Miscellaneous: Infusion related reaction (11% to 24%)
1% to 10%:
Cardiovascular: Peripheral edema (5% to 6%), chest discomfort (≤5%), syncope (≤2%)
Central nervous system: Vertigo (≤6%), dysesthesia (3%), rigors (≤3%), drowsiness (≤2%)
Dermatologic: Dermatitis (≤7%), pruritus (≤4%), urticaria (≤2%), thermal injury (1%), night sweats (≤1%), xeroderma (≤1%)
Endocrine & metabolic: Menstrual disease (≤5%), amenorrhea (≤2%), ovarian cyst (≤2%), weight changes (≤2%)
Gastrointestinal: Diarrhea (10%), tooth infection (≤9%), dyspepsia (≤5%), abdominal pain (≤4%), constipation (≤4%), toothache (≤4%), flatulence (≤3%), aphthous stomatitis (≤2%), cholelithiasis (≤1%), gingival disease (infection: 1%)
Genitourinary: Vaginal infection (≤10%), vaginitis (≤10%), urinary frequency (≤9%), dysmenorrhea (2% to 6%), urinary incontinence (≤4%)
Hematologic & oncologic: Hematoma (1%)
Hepatic: Increased serum transaminases (≤5%)
Hypersensitivity: Hypersensitivity reaction (acute: 2% to 4%; serious acute: ≤1%; delayed: ≤5%)
Immunologic: Antibody development (9% to 10%)
Infection: Herpes virus infection (≤8%), viral infection (≤7%), serious infection (2% to 3%)
Local: Bleeding at injection site (≤3%)
Neuromuscular & skeletal: Muscle cramps (≤5%), tremor (1% to 3%), joint swelling (≤2%)
Respiratory: Sinusitis (≤8%), cough (≤7%), tonsillitis (≤7%), pharyngolaryngeal pain (≤6%), epistaxis (2%)
Miscellaneous: Limb injury (3%), laceration (2%)
<1%, postmarketing, and/or case reports: Acne vulgaris, agitation, anaphylactoid reaction, anaphylaxis, anemia, angina pectoris, appendicitis, aspergillosis (bronchopulmonary), decreased hemoglobin (mild, transient), dizziness, dyspnea, erythema, exacerbation of Crohn’s disease, fever, flushing, gastroenteritis (cryptosporidial), hemolytic anemia, hepatic failure, hepatitis (cytomegalovirus), hepatotoxicity, herpes simplex encephalitis, hypotension, immune reconstitution syndrome, increased serum bilirubin, infection (Burkholderia cepacia), JC virus infection, joint stiffness, lethargy, leukocytosis, meningitis (herpes), nasopharyngitis, opportunistic infection (including bronchopulmonary infections, meningitis, and progressive multifocal leukoencephalopathy [PML]), muscle spasm, myasthenia, nail disease (onychorrhexis), paresis, pericarditis (case report), petechiae, pharyngitis, pneumonia (includes pneumonia caused by Pneumocystis jirovecii and varicella), psychomotor disturbance (hyperactivity), pulmonary infection (Mycobacterium avium intracellulare), suicidal ideation, tachycardia, thrombocytopenia, thrombophlebitis, vasodilation
Warnings/Precautions
Concerns related to adverse effects:
- Hepatotoxicity: Hepatotoxicity, including acute liver failure requiring transplant, has been reported with use. Signs of hepatotoxicity, including transaminase and bilirubin elevation occurred as early as 6 days after the first dose or following multiple doses; may recur with treatment rechallenge; discontinue use with jaundice or signs/symptoms of hepatic injury.
- Herpes infection: Serious, life-threatening herpes infections, including fatalities (herpes encephalitis or herpes meningitis infections caused by herpes simplex and varicella zoster viruses) have occurred within a few months to several years of natalizumab treatment. Monitor patients for signs/symptoms of meningitis and encephalitis. In the presence of herpes encephalitis or meningitis, discontinue therapy. Acute retinal necrosis (ARN), a fulminant viral infection of the retina caused by the family of herpes viruses (eg, varicella zoster, herpes simplex virus) has also been observed during natalizumab treatment. Some cases occurred in patients with CNS herpes infections (eg, herpes meningitis, encephalitis); serious cases may result in blindness. Following diagnosis of ARN, consider discontinuation of natalizumab.
- Hypersensitivity/antibody formation: Hypersensitivity reactions including serious systemic reactions (eg, anaphylaxis) have occurred in <1% of patients. Symptoms may include dizziness, fever, flushing, rigors, hypotension, dyspnea, chest pain, nausea, pruritus, rash, and urticaria and typically occur within 2 hours of starting the infusion. Reactions are generally associated with antibodies to natalizumab; consider the possibility of antibodies in patients who have hypersensitivity reactions. Patients with an extended interruption in therapy following a short exposure (1 to 2 infusions) to natalizumab may be at an increased risk for developing anti-natalizumab antibodies and/or hypersensitivity reactions following reinitiation of therapy. If a hypersensitivity reaction occurs, discontinue use; patients who have developed hypersensitivity reactions should not be re-treated. Antibody formation (which occurs in about 10% of patients) is associated with a decrease in natalizumab levels and a decrease in the efficacy of natalizumab. Antibody testing should be performed in any patient when there is a suspicion of persistent antibodies and should be considered in patients that resume therapy following a period of dosage interruption.
- Immune reconstitution inflammatory syndrome (IRIS): IRIS has been reported in patients after discontinuing natalizumab due to PML. In most cases, this occurred within days to weeks after plasma exchange was used in an attempt to remove natalizumab. IRIS is a rare condition which is characterized by severe inflammation during or following immune system recovery, which can result in a decline in patient condition, including neurological symptoms and death.
- Infections: Use may be associated with an increased risk of infections, including opportunistic infections and serious herpes infections (rare, postmarketing reports; concurrent use of antineoplastic, immunosuppressant [including short-course corticosteroids], or immunomodulating agents may increase this risk); discontinue therapy until successful resolution of the infection.
- Lab test abnormalities: Reversible increases in circulating lymphocytes, monocytes, eosinophils, basophils, and nucleated red blood cells may occur; changes persist during natalizumab exposure but usually return to baseline within 16 weeks after the last dose. Mild transient decreases in hemoglobin levels may also occur.
- Progressive multifocal leukoencephalopathy: [US Boxed Warning]: Natalizumab increases the risk of developing fatal or disabling progressive multifocal leukoencephalopathy (PML, an opportunistic viral infection of the brain caused by the JC virus). Risk factors for development of PML include duration of therapy (especially >2 years), prior use of immunosuppressants (eg, azathioprine, cyclophosphamide, methotrexate, mitoxantrone, mycophenolate), and the presence of anti JC virus antibodies. Monitor for any new signs/symptoms suggestive of PML; immediately withhold treatment at the first sign or symptom suggesting PML. For diagnosis of PML, an evaluation should include a gadolinium-enhanced MRI scan of the brain and (if indicated) analysis of CSF for JCV DNA. Retrospective analyses suggest risk of developing PML may be associated with relative serum anti-JCV antibody levels compared to a calibrator as measured by ELISA. Cases of PML have been diagnosed based on MRI findings and the detection of JCV DNA in the CSF without specific PML signs/symptoms (including progressive weakness on one side of the body, limb clumsiness, visual disturbance, changes in thinking, memory, personality or orientation) reported. Use should ordinarily be avoided in patients who are significantly immunocompromised or receiving chronic immunosuppressant or immunomodulatory therapy. For an early diagnosis of PML, consider periodic monitoring with an MRI scan for radiographic signs of PML. Anti-JCV antibody testing prior to or during treatment may be considered; testing should not be used to diagnose PML and should not be performed for at least 2 weeks after plasma exchange. Patients who are anti-JCV antibody negative are still at risk for developing PML, although the risk is lower; therefore, patients with a negative anti-JCV antibody test result should be retested periodically. A brain MRI scan (baseline) should be obtained prior to initiating therapy in MS patients and should be considered in patients with Crohn disease. PML has also been reported following discontinuation in patients who did not have findings suggestive of PML at the time of discontinuation; patients should be monitored for signs and symptoms of PML for at least 6 months after discontinuation of therapy.
Disease-related concerns:
- Crohn disease: Natalizumab should not be used in combination with immunosuppressants or tumor necrosis factor (TNF) inhibitors in patients with Crohn disease; aminosalicylates may be used concurrently with natalizumab. For patients who begin treatment while on chronic oral corticosteroids, begin tapering oral steroids when the onset of natalizumab therapeutic benefit is observed; discontinue use if patient cannot be tapered off of oral corticosteroids within 6 months of therapy initiation. If additional concomitant corticosteroids are required and exceed 3 months/year (in addition to initial corticosteroid taper), consider discontinuing therapy.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
- Immunizations: There are no data available concerning the effect of vaccination or secondary transmission of infection by live vaccines in patients receiving natalizumab.
Other warnings/precautions:
- Appropriate use: Use should be restricted to patients with inadequate response to or intolerant of other therapies for Crohn disease or multiple sclerosis. Carefully evaluate the overall benefit to risk in patients that develop persistent antibodies to natalizumab.
- Discontinuation of therapy: Cases of rebound syndrome (clinical and radiological signs of severe exacerbation beyond what was expected) have been reported; may occur within the first 24 weeks after stopping natalizumab treatment in patients with multiple sclerosis of varying severity and duration. In some cases, relapses have occurred despite the initiation of other disease-modifying therapies. Patients who experience rebound symptoms may not return to the functional status attained during treatment with natalizumab. Monitor for development of severe increase in disability, especially during the first 12 weeks following discontinuation and begin appropriate treatment as needed. Initiating fingolimod within 12 weeks after discontinuation of natalizumab may prevent relapse (AAN [Rae-Grant 2019]; Clerico 2017; Sellner 2019).
- REMS program: [US Boxed Warning]: Access is restricted through a REMS program called the TOUCH Prescribing Program; prescribers and pharmacies must be certified with the Tysabri Outreach Unified Commitment to Health (TOUCH) Prescribing Program. Patients must also be enrolled in the TOUCH Prescribing Program (800-456-2255) to receive natalizumab (MS-TOUCH for multiple sclerosis or CD-TOUCH for Crohn disease).
Monitoring Parameters
Symptoms of hepatotoxicity (eg, elevated serum transaminases, bilirubin); hypersensitivity reactions during, and for 1 hour after, infusion; symptoms of persistent antibody-positivity (eg, anxiety, dizziness, dyspnea, feeling cold, flushing, headache, hypertension, myalgia, nausea, pruritus, pyrexia, rigors, tachycardia, tremor, urticaria or, vomiting); signs/symptoms of meningitis and encephalitis; signs/symptoms of acute retinal necrosis.
Radiographic signs of PML periodically; antibody testing is recommended if persistent antibodies are suspected and repeated in 3 months in all patients with documented positivity on initial test; consider antibody testing in patients that resume therapy following a period of dosage interruption.
Baseline brain MRI scan; if PML is suspected, obtain gadolinium-enhanced brain MRI scan and CSF analysis for JC viral DNA. Evaluate for signs or symptoms of progressive multifocal leukoencephalopathy during treatment and for 6 months after discontinuation. Note: Transient and reversible leukocytosis (excluding neutrophils) and mildly reduced hemoglobin may occur with treatment and may require ~4 months for return to baseline values after the last dose; anti-JCV antibody (prior to therapy and periodically during therapy).
Pregnancy
Pregnancy Considerations
Natalizumab crosses the placenta (Haghikia 2014; Proschmann 2018).
Natalizumab is a humanized monoclonal antibody (IgG4). Placental transfer of human IgG is dependent upon the IgG subclass, maternal serum concentrations, birth weight, and gestational age, generally increasing as pregnancy progresses. The lowest exposure would be expected during the period of organogenesis (Palmeira 2012; Pentsuk 2009).
Outcome information related to the use of natalizumab in pregnancy is available from pregnancy registries and systematic reviews in which most females discontinued use once pregnancy was detected. The risk of adverse outcomes (such as spontaneous abortion or birth defects) was not consistent between studies (Ebrahimi 2015; Friend 2016; Peng 2019; Portaccio 2018a). Hematological abnormalities in the newborn, such as anemia and thrombocytopenia, have been noted following maternal use during the third trimester (Haghikia 2014; Proschmann 2018).
In general, disease-modifying therapies for multiple sclerosis (MS) are stopped prior to a planned pregnancy and not initiated during pregnancy, except in females at high risk of MS activity (AAN [Rae-Grant 2018]). Consider use of agents other than natalizumab for females at high risk of disease reactivation who are planning a pregnancy (ECTRIMS/EAN [Montalban 2018]). Clinical rebound (new neurologic symptoms and increased lesions) has been reported when natalizumab was discontinued prior to or during pregnancy. Some studies suggest that continuing treatment until pregnancy is confirmed then restarting soon after delivery may prevent relapse in high-risk patients (De Giglio 2015; Kleerekooper 2017; Portaccio 2018b; Vukusic 2015). Delaying pregnancy is recommended for females with persistent high disease activity; however, when disease-modifying therapy is needed in these patients, natalizumab may be continued during pregnancy following a full discussion of potential risks (ECTRIMS/EAN [Montalban 2018]).
Inflammatory bowel disease is associated with adverse pregnancy outcomes including an increased risk of miscarriage, premature delivery, delivery of a low birth weight infant, and poor maternal weight gain. Management of maternal disease should be optimized prior to pregnancy. Treatment decreases disease flares, disease activity, and the incidence of adverse pregnancy outcomes. When treatment for inflammatory bowel disease is needed in pregnant women, appropriate biologic therapy can be continued without interruption. Serum levels should be evaluated prior to conception and optimized to avoid subtherapeutic concentrations or high levels which may increase placental transfer. Dosing can be adjusted so delivery occurs at the lowest serum concentration. For natalizumab, the final injection can be given 4 to 6 weeks prior to the estimated date of delivery, then continued 48 hours' postpartum (Mahadevan 2019).
Patient Education
What is this drug used for?
- It is used to treat MS (multiple sclerosis).
- It is used to treat Crohn disease.
Frequently reported side effects of this drug
- Abdominal pain
- Diarrhea
- Back pain
- Painful extremities
- Loss of strength and energy
- Muscle cramps
- Common cold symptoms
- Joint pain
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Infection
- Progressive multifocal leukoencephalopathy like confusion, depression, trouble with memory, behavioral changes, change in strength on one side, trouble speaking, change in balance, or vision changes.
- Urinary tract infection like blood in the urine, burning or pain when passing urine, passing a lot of urine, fever, lower abdominal pain, or pelvic pain.
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Depression
- Severe nausea
- Vomiting
- Vaginal pain, itching, and discharge
- Dizziness
- Flushing
- Shortness of breath
- Chest pain
- Menstrual changes
- Swelling of arms of legs
- Passing out
- Vision changes
- Eye pain
- Eye redness
- Severe headache
- Confusion
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.