Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
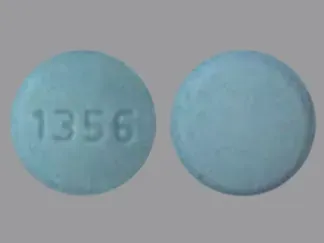
Bystolic: 2.5 mg, 5 mg, 10 mg, 20 mg [contains fd&c blue #2 aluminum lake, fd&c yellow #6 aluminum lake]
Pharmacology
Mechanism of Action
Highly-selective inhibitor of beta1-adrenergic receptors; at doses ≤10 mg nebivolol preferentially blocks beta1-receptors. Nebivolol, unlike other beta-blockers, also produces an endothelium-derived nitric oxide-dependent vasodilation resulting in a reduction of systemic vascular resistance.
Pharmacokinetics/Pharmacodynamics
Absorption
Rapid
Distribution
Vd: 8 to 12 L/kg
Metabolism
Hepatic; via glucuronidation and CYP2D6; extensive first-pass metabolism to multiple active metabolites with variable activity
Excretion
Urine (extensive metabolizers: 38%; poor metabolizers: 67%; <0.5% of total dose as unchanged drug); feces (extensive metabolizers: 44%; poor metabolizers: 13%; <0.5% of total dose as unchanged drug)
Time to Peak
1.5 to 4 hours
Half-Life Elimination
Terminal: 12 hours (extensive metabolizers) or 19 hours (poor metabolizers); up to 32 hours has been reported in poor metabolizers (Mangrella 1998).
Protein Binding
~98%, primarily to albumin
Use in Specific Populations
Special Populations: Renal Function Impairment
Clearance was unchanged in patients with mild renal impairment and was reduced negligibly in patients with moderate renal impairment. However, clearance was reduced by 53% in patients with severe renal impairment.
Special Populations: Hepatic Function Impairment
Cmax increases 3-fold, AUC increases 10-fold, and apparent clearance decreases 86% in patients with moderate hepatic impairment.
Use: Labeled Indications
Hypertension: Management of hypertension. Note: Beta-blockers are not recommended as first-line therapy (ACC/AHA [Whelton 2017]).
Contraindications
Hypersensitivity to nebivolol or any component of the formulation; severe bradycardia; heart block greater than first-degree (except in patients with a functioning artificial pacemaker); cardiogenic shock; decompensated heart failure; sick sinus syndrome (unless a permanent pacemaker is in place); severe hepatic impairment (Child-Pugh class C)
Documentation of allergenic cross-reactivity for beta-blockers is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Canadian labeling: Additional contraindications (not in US labeling): Severe peripheral arterial circulatory disorders; sinoatrial block; rare hereditary conditions of Galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption
Dosage and Administration
Dosing: Adult
Hypertension (alternative agent): Oral: Initial: 5 mg once daily; titrate as needed at 2-week intervals based on patient response to a maximum dose of 40 mg once daily (ACC/AHA [Whelton 2017]).
Dosing: Geriatric
Refer to adult dosing.
Administration
Oral: May be administered with or without food.
Storage
Store at 20°C to 25°C (68°F to 77°F). Protect from light.
Nebivolol Images
Drug Interactions
Acetylcholinesterase Inhibitors: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Alfuzosin: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Alpha1-Blockers: Beta-Blockers may enhance the orthostatic hypotensive effect of Alpha1-Blockers. The risk associated with ophthalmic products is probably less than systemic products. Monitor therapy
Alpha2-Agonists: May enhance the AV-blocking effect of Beta-Blockers. Sinus node dysfunction may also be enhanced. Beta-Blockers may enhance the rebound hypertensive effect of Alpha2-Agonists. This effect can occur when the Alpha2-Agonist is abruptly withdrawn. Management: Closely monitor heart rate during treatment with a beta blocker and clonidine. Withdraw beta blockers several days before clonidine withdrawal when possible, and monitor blood pressure closely. Recommendations for other alpha2-agonists are unavailable. Exceptions: Apraclonidine. Consider therapy modification
Amifostine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Amifostine. Management: When amifostine is used at chemotherapy doses, blood pressure lowering medications should be withheld for 24 hours prior to amifostine administration. If blood pressure lowering therapy cannot be withheld, amifostine should not be administered. Consider therapy modification
Aminoquinolines (Antimalarial): May decrease the metabolism of Beta-Blockers. Monitor therapy
Amiodarone: May enhance the bradycardic effect of Beta-Blockers. Possibly to the point of cardiac arrest. Amiodarone may increase the serum concentration of Beta-Blockers. Monitor therapy
Amphetamines: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Antipsychotic Agents (Phenothiazines): May enhance the hypotensive effect of Beta-Blockers. Beta-Blockers may decrease the metabolism of Antipsychotic Agents (Phenothiazines). Antipsychotic Agents (Phenothiazines) may decrease the metabolism of Beta-Blockers. Monitor therapy
Antipsychotic Agents (Second Generation [Atypical]): Blood Pressure Lowering Agents may enhance the hypotensive effect of Antipsychotic Agents (Second Generation [Atypical]). Monitor therapy
Barbiturates: May decrease the serum concentration of Beta-Blockers. Monitor therapy
Barbiturates: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Benperidol: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Beta2-Agonists: Beta-Blockers (Beta1 Selective) may diminish the bronchodilatory effect of Beta2-Agonists. Of particular concern with nonselective beta-blockers or higher doses of the beta1 selective beta-blockers. Monitor therapy
Bradycardia-Causing Agents: May enhance the bradycardic effect of other Bradycardia-Causing Agents. Monitor therapy
Brigatinib: May diminish the antihypertensive effect of Antihypertensive Agents. Brigatinib may enhance the bradycardic effect of Antihypertensive Agents. Monitor therapy
Brimonidine (Topical): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Bromperidol: Blood Pressure Lowering Agents may enhance the hypotensive effect of Bromperidol. Bromperidol may diminish the hypotensive effect of Blood Pressure Lowering Agents. Avoid combination
Bupivacaine: Beta-Blockers may increase the serum concentration of Bupivacaine. Monitor therapy
Calcium Channel Blockers (Nondihydropyridine): May enhance the hypotensive effect of Beta-Blockers. Bradycardia and signs of heart failure have also been reported. Calcium Channel Blockers (Nondihydropyridine) may increase the serum concentration of Beta-Blockers. Exceptions: Bepridil. Monitor therapy
Cardiac Glycosides: Beta-Blockers may enhance the bradycardic effect of Cardiac Glycosides. Monitor therapy
Ceritinib: Bradycardia-Causing Agents may enhance the bradycardic effect of Ceritinib. Management: If this combination cannot be avoided, monitor patients for evidence of symptomatic bradycardia, and closely monitor blood pressure and heart rate during therapy. Exceptions are discussed in separate monographs. Consider therapy modification
Cholinergic Agonists: Beta-Blockers may enhance the adverse/toxic effect of Cholinergic Agonists. Of particular concern are the potential for cardiac conduction abnormalities and bronchoconstriction. Monitor therapy
CYP2D6 Inhibitors (Moderate): May increase the serum concentration of Nebivolol. Monitor therapy
CYP2D6 Inhibitors (Strong): May increase the serum concentration of Nebivolol. Monitor therapy
Dexmethylphenidate: May diminish the therapeutic effect of Antihypertensive Agents. Monitor therapy
Diazoxide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Dipyridamole: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Disopyramide: May enhance the bradycardic effect of Beta-Blockers. Beta-Blockers may enhance the negative inotropic effect of Disopyramide. Monitor therapy
Dronedarone: May enhance the bradycardic effect of Beta-Blockers. Dronedarone may increase the serum concentration of Beta-Blockers. This likely applies only to those agents that are metabolized by CYP2D6. Management: Use lower initial beta-blocker doses; adequate tolerance of the combination, based on ECG findings, should be confirmed prior to any increase in beta-blocker dose. Consider therapy modification
DULoxetine: Blood Pressure Lowering Agents may enhance the hypotensive effect of DULoxetine. Monitor therapy
EPINEPHrine (Nasal): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Nasal). Monitor therapy
EPINEPHrine (Oral Inhalation): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Oral Inhalation). Monitor therapy
Epinephrine (Racemic): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of Epinephrine (Racemic). Monitor therapy
EPINEPHrine (Systemic): Beta-Blockers (Beta1 Selective) may diminish the therapeutic effect of EPINEPHrine (Systemic). Monitor therapy
Ergot Derivatives: Beta-Blockers may enhance the vasoconstricting effect of Ergot Derivatives. Exceptions: Nicergoline. Consider therapy modification
Fexinidazole [INT]: Bradycardia-Causing Agents may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Fingolimod: Beta-Blockers may enhance the bradycardic effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and beta-blockers if possible. If coadministration is necessary, patients should have overnight continuous ECG monitoring conducted after the first dose of fingolimod. Monitor patients for bradycardia. Consider therapy modification
Floctafenine: May enhance the adverse/toxic effect of Beta-Blockers. Avoid combination
Grass Pollen Allergen Extract (5 Grass Extract): Beta-Blockers may enhance the adverse/toxic effect of Grass Pollen Allergen Extract (5 Grass Extract). More specifically, Beta-Blockers may inhibit the ability to effectively treat severe allergic reactions to Grass Pollen Allergen Extract (5 Grass Extract) with epinephrine. Some other effects of epinephrine may be unaffected or even enhanced (e.g., vasoconstriction) during treatment with Beta-Blockers. Consider therapy modification
Herbs (Hypertensive Properties): May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Herbs (Hypotensive Properties): May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Hypotension-Associated Agents: Blood Pressure Lowering Agents may enhance the hypotensive effect of Hypotension-Associated Agents. Monitor therapy
Insulins: Beta-Blockers may enhance the hypoglycemic effect of Insulins. Monitor therapy
Ivabradine: Bradycardia-Causing Agents may enhance the bradycardic effect of Ivabradine. Monitor therapy
Lacosamide: Bradycardia-Causing Agents may enhance the AV-blocking effect of Lacosamide. Monitor therapy
Levodopa-Containing Products: Blood Pressure Lowering Agents may enhance the hypotensive effect of Levodopa-Containing Products. Monitor therapy
Lidocaine (Systemic): Beta-Blockers may increase the serum concentration of Lidocaine (Systemic). Monitor therapy
Lidocaine (Topical): Beta-Blockers may increase the serum concentration of Lidocaine (Topical). Monitor therapy
Lormetazepam: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Mepivacaine: Beta-Blockers may increase the serum concentration of Mepivacaine. Monitor therapy
Methacholine: Beta-Blockers may enhance the adverse/toxic effect of Methacholine. Monitor therapy
Methoxyflurane: May enhance the hypotensive effect of Beta-Blockers. Monitor therapy
Methylphenidate: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Midodrine: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Molsidomine: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Naftopidil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicergoline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Nicorandil: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
NIFEdipine: May enhance the hypotensive effect of Beta-Blockers. NIFEdipine may enhance the negative inotropic effect of Beta-Blockers. Monitor therapy
Nitroprusside: Blood Pressure Lowering Agents may enhance the hypotensive effect of Nitroprusside. Monitor therapy
Nonsteroidal Anti-Inflammatory Agents: May diminish the antihypertensive effect of Beta-Blockers. Monitor therapy
Obinutuzumab: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Management: Consider temporarily withholding blood pressure lowering medications beginning 12 hours prior to obinutuzumab infusion and continuing until 1 hour after the end of the infusion. Consider therapy modification
Opioids (Anilidopiperidine): May enhance the bradycardic effect of Beta-Blockers. Opioids (Anilidopiperidine) may enhance the hypotensive effect of Beta-Blockers. Monitor therapy
Pentoxifylline: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Pholcodine: Blood Pressure Lowering Agents may enhance the hypotensive effect of Pholcodine. Monitor therapy
Phosphodiesterase 5 Inhibitors: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Propafenone: May increase the serum concentration of Beta-Blockers. Propafenone possesses some independent beta blocking activity. Monitor therapy
Prostacyclin Analogues: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Quinagolide: May enhance the hypotensive effect of Blood Pressure Lowering Agents. Monitor therapy
Regorafenib: May enhance the bradycardic effect of Beta-Blockers. Monitor therapy
Reserpine: May enhance the hypotensive effect of Beta-Blockers. Monitor therapy
Rifamycin Derivatives: May decrease the serum concentration of Beta-Blockers. Exceptions: Rifabutin. Monitor therapy
Rivastigmine: May enhance the bradycardic effect of Beta-Blockers. Avoid combination
Ruxolitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Management: Ruxolitinib Canadian product labeling recommends avoiding use with bradycardia-causing agents to the extent possible. Monitor therapy
Selective Serotonin Reuptake Inhibitors: May increase the serum concentration of Beta-Blockers. Exceptions: Citalopram; Escitalopram; FluvoxaMINE. Monitor therapy
Siponimod: Bradycardia-Causing Agents may enhance the bradycardic effect of Siponimod. Management: Avoid coadministration of siponimod with drugs that may cause bradycardia. Consider therapy modification
Sulfonylureas: Beta-Blockers may enhance the hypoglycemic effect of Sulfonylureas. Cardioselective beta-blockers (eg, acebutolol, atenolol, metoprolol, and penbutolol) may be safer than nonselective beta-blockers. All beta-blockers appear to mask tachycardia as an initial symptom of hypoglycemia. Ophthalmic beta-blockers are probably associated with lower risk than systemic agents. Monitor therapy
Terlipressin: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Theophylline Derivatives: Beta-Blockers (Beta1 Selective) may diminish the bronchodilatory effect of Theophylline Derivatives. Management: Monitor for reduced theophylline efficacy during concomitant use with any beta-blocker. Beta-1 selective agents are less likely to antagonize theophylline than nonselective agents, but selectivity may be lost at higher doses. Monitor therapy
Tofacitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Yohimbine: May diminish the antihypertensive effect of Antihypertensive Agents. Monitor therapy
Test Interactions
May lead to false-positive aldosterone/renin ratio (ARR) (Funder 2016)
Adverse Reactions
1% to 10%:
Cardiovascular: Peripheral edema (1%), bradycardia (≤1%), chest pain (≤1%)
Central nervous system: Headache (6% to 9%), fatigue (dose-related; 2% to 5%), dizziness (2% to 4%), insomnia (1%), paresthesia
Dermatologic: Skin rash (≤1%)
Endocrine & metabolic: Decreased HDL cholesterol, hypercholesterolemia, increased serum triglycerides, increased uric acid
Gastrointestinal: Diarrhea (dose-related; 2% to 3%), nausea (1% to 3%), abdominal pain
Hematologic & oncologic: Decreased platelet count
Neuromuscular & skeletal: Weakness
Renal: Increased blood urea nitrogen
Respiratory: Dyspnea (≤1%)
<1%, postmarketing, and/or case reports: Acute pulmonary edema, acute renal failure, angioedema, atrioventricular block (second and third degree), bronchospasm, claudication, dermatological disease, drowsiness, erectile dysfunction, hepatic insufficiency, hypersensitivity angiitis, hypersensitivity reaction, increased serum ALT, increased serum AST, increased serum bilirubin, myocardial infarction, peripheral ischemia, pruritus, psoriasis, Raynaud's phenomenon, syncope, thrombocytopenia, urticaria, vertigo, vomiting
Warnings/Precautions
Concerns related to adverse effects:
- Anaphylactic reactions: Use caution with history of severe anaphylaxis to a variety of allergens; patients taking beta-blockers may become more sensitive to repeated challenges. Treatment of anaphylaxis (eg, epinephrine) in patients taking beta-blockers may be ineffective or promote undesirable effects.
Disease-related concerns:
- Bronchospastic disease: In general, patients with bronchospastic disease should not receive beta-blockers; for patients with bronchospastic disease who do not respond to or cannot tolerate other therapies, initial low doses of beta1-selective nebivolol may be employed and used cautiously with close monitoring. Ensure patient has an inhaled beta2-agonist immediately available.
- Diabetes: Use with caution in patients with diabetes mellitus; may potentiate hypoglycemia and/or mask signs and symptoms.
- Heart failure (HF): Note: Nebivolol has not been shown to reduce morbidity or mortality in the general HF population; only beta-blockers proven to reduce mortality (ie, bisoprolol, carvedilol, or extended-release metoprolol succinate) should be used in the treatment of heart failure. Use with caution in patients with compensated HF and monitor for a worsening of the condition. If condition worsens, consider temporary discontinuation or dosage reduction of nebivolol. Patients should be stabilized on HF regimen prior to initiation of beta-blocker. Beta-blocker therapy should be initiated at very low doses with gradual and very careful titration. Adjustment of other medications (ACE inhibitors and/or diuretics) may be required.
- Hepatic impairment: Use with caution in patients with hepatic impairment; dosage adjustment required with moderate impairment (Child-Pugh class B). Use is contraindicated in patients with Child-Pugh class C hepatic impairment.
- Myasthenia gravis: Use with caution in patients with myasthenia gravis.
- Peripheral vascular disease (PVD) and Raynaud disease: Can precipitate or aggravate symptoms of arterial insufficiency in patients with PVD and Raynaud disease. Use with caution and monitor for progression of arterial obstruction.
- Pheochromocytoma (untreated): Adequate alpha-blockade is required prior to use of any beta-blocker.
- Psoriasis: Beta-blocker use has been associated with induction or exacerbation of psoriasis, but cause and effect have not been firmly established.
- Renal impairment: Use with caution in patients with renal impairment; dosage adjustment required with severe renal impairment (CrCl <30 mL/minute). Nebivolol has not been evaluated in dialysis-dependent patients.
- Thyroid disease: May mask signs of hyperthyroidism (eg, tachycardia). If thyrotoxicosis is suspected, carefully manage and monitor; abrupt withdrawal may exacerbate symptoms of hyperthyroidism or precipitate thyroid storm.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Elderly: Bradycardia may be observed more frequently in elderly patients (>65 years of age); dosage reductions may be necessary.
Other warnings/precautions:
- Abrupt withdrawal: Beta-blocker therapy should not be withdrawn abruptly (particularly in patients with coronary artery disease), but gradually tapered over 1-2 weeks to avoid acute tachycardia, hypertension, and/or ischemia. Severe exacerbation of angina, ventricular arrhythmias, and myocardial infarction (MI) have been reported following abrupt withdrawal of beta-blocker therapy. Temporary but prompt resumption of beta-blocker therapy may be indicated with worsening of angina or acute coronary insufficiency.
- Major surgery: Chronic beta-blocker therapy should not be routinely withdrawn prior to major surgery.
Monitoring Parameters
Blood pressure, ECG; serum glucose (in diabetic patients)
Hypertension: The 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults (ACC/AHA [Whelton 2017]):
Confirmed hypertension and known CVD or 10-year ASCVD risk ≥10%: Target blood pressure <130/80 mm Hg is recommended.
Confirmed hypertension without markers of increased ASCVD risk: Target blood pressure <130/80 mm Hg may be reasonable.
Pregnancy
Pregnancy Considerations
Outcome information following maternal use of nebivolol in pregnancy is limited (Sullo 2015).
Exposure to beta-blockers during pregnancy may increase the risk for adverse events in the neonate. If maternal use of a beta-blocker is needed, fetal growth should be monitored during pregnancy and the newborn should be monitored for 48 hours after delivery for bradycardia, hypoglycemia, and respiratory depression (ESC [Regitz-Zagrosek 2018]).
Chronic maternal hypertension is also associated with adverse events in the fetus/infant. Chronic maternal hypertension may increase the risk of birth defects, low birth weight, premature delivery, stillbirth, and neonatal death. Actual fetal/neonatal risks may be related to duration and severity of maternal hypertension. Untreated chronic hypertension may also increase the risks of adverse maternal outcomes, including gestational diabetes, preeclampsia, delivery complications, stroke, and myocardial infarction (ACOG 203 2019).
When treatment of chronic hypertension in pregnancy is indicated, agents other than nebivolol are preferred (ACOG 203 2013; ESC [Regitz-Zagrosek 2018]; Magee 2014). Females with preexisting hypertension may continue their medication during pregnancy unless contraindications exist (ESC [Regitz-Zagrosek 2018]).
Patient Education
What is this drug used for?
- It is used to treat high blood pressure.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Headache
- Loss of strength and energy
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Low blood sugar like dizziness, headache, fatigue, feeling weak, shaking, fast heartbeat, confusion, increased hunger, or sweating.
- Dizziness
- Passing out
- Shortness of breath
- Excessive weight gain
- Swelling of arms or legs
- Slow heartbeat
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.