Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Concentrate, Oral:
predniSONE Intensol: 5 mg/mL (30 mL) [contains alcohol, usp; unflavored flavor]
Solution, Oral:
Generic: 5 mg/5 mL (120 mL, 500 mL)
Tablet, Oral:
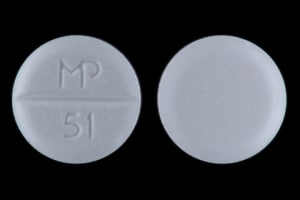
Deltasone: 20 mg [scored; contains fd&c yellow #10 aluminum lake, fd&c yellow #6 aluminum lake]
Generic: 1 mg, 2.5 mg, 5 mg, 10 mg, 20 mg, 50 mg
Tablet Delayed Release, Oral:
Rayos: 1 mg, 2 mg, 5 mg
Tablet Therapy Pack, Oral:
Generic: 10 mg (21 ea, 48 ea); 5 mg (21 ea, 48 ea)
Pharmacology
Mechanism of Action
Decreases inflammation by suppression of migration of polymorphonuclear leukocytes and reversal of increased capillary permeability; suppresses the immune system by reducing activity and volume of the lymphatic system; suppresses adrenal function at high doses. Antitumor effects may be related to inhibition of glucose transport, phosphorylation, or induction of cell death in immature lymphocytes. Antiemetic effects are thought to occur due to blockade of cerebral innervation of the emetic center via inhibition of prostaglandin synthesis.
Pharmacokinetics/Pharmacodynamics
Absorption
50% to 90% (may be altered in hepatic failure, chronic renal failure, inflammatory bowel disease, hyperthyroidism, and in the elderly) (Frey 1990)
Metabolism
Hepatic to metabolite prednisolone (active)
Excretion
Urine (as conjugates)
Time to Peak
Oral: Immediate-release tablet: 2 hours; Delayed-release tablet: 6 to 6.5 hours
Half-Life Elimination
2 to 3 hours
Protein Binding
Concentration dependent: <50% (Frey 1990)
Use in Specific Populations
Special Populations: Hepatic Function Impairment
Prednisone is inactive and must be metabolized by the liver to prednisolone. This conversion may be impaired in patients with liver disease; however, prednisolone levels are observed to be higher in patients with severe liver failure than in normal patients.
Use: Labeled Indications
Anti-inflammatory or immunosuppressant agent in the treatment of a variety of diseases, including allergic, hematologic (eg, immune thrombocytopenia, warm autoimmune hemolytic anemia), dermatologic, GI, inflammatory, ophthalmic, neoplastic, rheumatic (eg, acute gout flare, vasculitis, dermatomyositis, mixed cryoglobulinemia syndrome, polyarteritis nodosa, polymyositis, polymyalgia rheumatica, rheumatoid arthritis, systemic lupus erythematosus), autoimmune, nervous system (eg, acute exacerbations of multiple sclerosis), renal, respiratory, and endocrine (eg, primary or secondary adrenocorticoid deficiency); solid organ rejection (acute/chronic).
Use: Off Label
Bell palsy, new onsetbyes
Data from a randomized, double-blind, placebo-controlled trial demonstrated that prednisone, given as early treatment for new-onset Bell palsy, resulted in significant improvement in facial grade at recovery compared to placebo Austin 1993.
Based on the American Academy of Otolaryngology-Head and Neck Surgery clinical practice guideline on Bell palsy, prednisone (or prednisolone), when initiated within 72 hours of symptom onset, is effective and recommended in the management of Bell palsy.
Chronic obstructive pulmonary disease, acute exacerbationyes
Based on the Global Initiative for Chronic Obstructive Lung Disease's global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2019 report, prednisone is recommended for acute exacerbation of chronic obstructive pulmonary disease. Short-term treatment with systemic corticosteroids has been shown to reduce recovery time, risk of early relapse, treatment failure, and length of hospital stay, as well as improve lung function; however, long-term use is associated with significant adverse effects.
Chronic spontaneous urticaria, acute exacerbationyes
Based on the European Academy of Allergology and Clinical Immunology/Global Asthma and Allergy European Network/European Dermatology Forum/World Allergy Organization guidelines for the management of urticaria, prednisone given for acute exacerbation of chronic spontaneous urticaria is effective and recommended in the management of this condition. Long-term use is not recommended.
Duchenne muscular dystrophybyes
Data from a meta-analysis, randomized controlled trials, and retrospective cohort studies support the use of prednisone for increasing muscular strength and function, improving pulmonary function, decreasing timed motor function, delaying development of scoliosis or cardiomyopathy, and delaying surgery for scoliosis in patients with Duchenne muscular dystrophy Bonifati 2000, King 2007, Markham 2008, Matthews 2016, Schram 2013.
Based on the American Academy of Neurology guidelines for corticosteroid treatment of Duchenne muscular dystrophy, prednisone probably improves muscle strength and pulmonary function and possibly improves timed motor function, slows the development of scoliosis, reduces the need for scoliosis surgery by age 18, and delays the onset of cardiomyopathy.
Giant cell arteritis, treatmentyes
Based on the British Society for Rheumatology/British Health Professionals in Rheumatology guidelines for the management of giant cell arteritis, high-dose glucocorticoids (eg, prednisone) are effective and recommended to improve visual prognosis in giant cell arteritis, when initiated immediately after suspected or confirmed diagnosis.
Graft-versus-host disease, acute, treatmentc
Data from a review by the American Society of Blood and Marrow Transplantation support the use of prednisone for grade II or higher acute graft-versus-host disease Martin 2012.
Hepatitis, autoimmunebyes
Data from randomized, double-blind, prospective studies support the use of prednisone alone or in combination with azathioprine for the treatment of autoimmune hepatitis Murray-Lyon 1973, Soloway 1972.
Based on the American Association for the Study of Liver Diseases guidelines for the diagnosis and management of autoimmune hepatitis, the use of prednisone alone or in combination with azathioprine is an effective and recommended regimen in the management of this condition.
Minimal change disease, treatmentc
Clinical experience suggests the utility of prednisone in the treatment of minimal change disease Hogan 2013, Meyrier 2019, Vivarelli 2017.
Multiple myeloma (previously untreated; transplant-ineligible)a
Data from a large, multicenter, randomized phase 3 study support the use of prednisone as a treatment component (in combination with daratumumab, bortezomib, and melphalan) in the management of previously untreated multiple myeloma in patients who are ineligible for autologous stem cell transplant Mateos 2018. Data from a randomized phase 3 study support the use of prednisone as a treatment component (in combination with bortezomib and melphalan) for management of symptomatic multiple myeloma in previously untreated patients who are not candidates for transplantation San Miguel 2008. Data from another large randomized phase 3 study support the use of prednisone as a treatment component (in combination with melphalan and thalidomide) for management of multiple myeloma in elderly patients who are ineligible for transplant Facon 2007. A multicenter, randomized, parallel study supports the use of prednisone (in combination with melphalan) as a treatment option in previously untreated elderly patients with multiple myeloma who are not transplant candidates Facon 2006.
Myasthenia gravis, crisisc
Data from a review support the use of prednisone as an adjunctive treatment of myasthenic crisis Lacomis 2005.
Pericarditis, acuteyes
Based on the European Society of Cardiology guidelines for the diagnosis and management of pericardial disease, prednisone is recommended as an alternative for the treatment of acute pericarditis when there are contraindications or incomplete response to aspirin/nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine.
Pneumocystis pneumonia, adjunctive therapy for moderate to severe diseasecyes
Data from a small, retrospective, single-center study evaluating the use of corticosteroids in adults with severe non-HIV-related Pneumocystis jirovecii pneumonia suggest that high-dose corticosteroid therapy, as an adjunct to antimicrobial therapy, shortens mechanical ventilation duration, ICU stay, and supplemental oxygen duration Pareja 1998.
Based on the US Department of Health and Human Services guidelines for prevention and treatment of opportunistic infections in adults and adolescents with HIV, prednisone is an effective and recommended treatment in the adjunctive treatment of pneumocystis pneumonia in HIV-infected patients.
Prostate cancer, metastatica
Data from two large randomized phase 3 studies support the use of prednisone (in combination with abiraterone) in the treatment of metastatic castration-resistant prostate cancer de Bono 2011, Ryan 2015. Data from a randomized phase 3 study support the use of prednisone (in combination with cabazitaxel) in the treatment of metastatic castration-resistant prostate cancer that has progressed during or following docetaxel-based therapy de Bono 2010. Data from a randomized phase 3 study support the use of prednisone (in combination with docetaxel) in the treatment of hormone-refractory metastatic prostate cancer Berthold 2008, Tannock 2004.
Takayasu arteritisyes
Based on the European League Against Rheumatism guidelines for the management of large vessel vasculitis, prednisone is effective and recommended for the induction of remission in active Takayasu arteritis.
Thyroiditis, subacuteyes
Based on the American Thyroid Association guidelines for the diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis, corticosteroids (eg, prednisone) are recommended for the treatment of subacute thyroiditis in patients who fail to respond to initial NSAID therapy or in patients with moderate to severe pain and/or thyrotoxic symptoms on initial presentation.
Tuberculosis, pulmonaryb
Data from a randomized, double-blind, placebo-controlled trial suggest the use of prednisone for the prevention of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS) is associated with a reduction in the incidence of IRIS in HIV-infected patients with CD4 count ≤100 cells/mm3 when used within the first 4 weeks of antiretroviral therapy initiation Meintjes 2018.
Contraindications
Hypersensitivity to prednisone or any component of the formulation; administration of live or live attenuated vaccines with immunosuppressive doses of prednisone; systemic fungal infections
Canadian labeling: Additional contraindications (not in US labeling): Herpes simplex of the eye, measles, or chickenpox (except when being used for short-term or emergency therapy); peptic ulcer; nonspecific ulcerative colitis; diverticulitis; viral or bacterial infection not controlled by anti-infectives
Documentation of allergenic cross-reactivity for corticosteroids is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Dosage and Administration
Dosing: Adult
Note: Dosing: Evidence to support an optimal dose and duration is lacking for most indications; recommendations provided are general guidelines only and primarily based on expert opinion. In general, glucocorticoid dosing should be individualized and the minimum effective dose/duration should be used. For select indications with weight-based dosing, consider using ideal body weight in obese patients, especially with longer durations of therapy (Erstad 2004; Furst 2019a). Hypothalamic-pituitary-adrenal (HPA) suppression: Although some patients may become HPA suppressed with lower doses or briefer exposure, some experts consider HPA-axis suppression likely in any adult receiving >20 mg/day (daytime dosing) or ≥5 mg per 24 hours (evening or night dosing) for >3 weeks or with Cushingoid appearance (Furst 2019b; Joseph 2016); do not abruptly discontinue treatment in these patients; dose tapering may be necessary (Cooper 2003).
Usual dosage range:
Oral: 10 to 60 mg/day given in a single daily dose or in 2 to 4 divided doses; Low dose: 2.5 to 10 mg/day; High dose: 1 to 1.5 mg/kg/day (usually not to exceed 80 to 100 mg/day).
The following dosing is from the commercially available tapered-dosage product (eg, dose pack containing 21 x 5 mg tablets):
Day 1: 30 mg on day 1 administered as 10 mg (2 tablets) at breakfast, 5 mg (1 tablet) at lunch, 5 mg (1 tablet) at dinner, and 10 mg (2 tablets) at bedtime.
Day 2: 25 mg on day 2 administered as 5 mg (1 tablet) at breakfast, 5 mg (1 tablet) at lunch, 5 mg (1 tablet) at dinner, and 10 mg (2 tablets) at bedtime.
Day 3: 20 mg on day 3 administered as 5 mg (1 tablet) at breakfast, 5 mg (1 tablet) at lunch, 5 mg (1 tablet) at dinner, and 5 mg (1 tablet) at bedtime.
Day 4: 15 mg on day 4 administered as 5 mg (1 tablet) at breakfast, 5 mg (1 tablet) at lunch, and 5 mg (1 tablet) at bedtime.
Day 5: 10 mg on day 5 administered as 5 mg (1 tablet) at breakfast and 5 mg (1 tablet) at bedtime.
Day 6: 5 mg on day 6 administered as 5 mg (1 tablet) at breakfast.
Indication-specific dosing:
Adrenal insufficiency, primary chronic (alternative agent): Note: In general, hydrocortisone is preferred. Use in conjunction with fludrocortisone. Dose is based on prednisolone equivalency.
Oral: 2.5 to 7.5 mg once daily (Bornstein 2016; Nieman 2019).
Allergic conditions:
Anaphylaxis (adjunct to epinephrine for prevention of late-phase/biphasic reaction): Note: Do not use for initial or sole treatment of anaphylaxis because corticosteroids do not result in the prompt relief of upper or lower airway obstruction or shock (AAAAI [Lieberman 2015]; EAACI [Muraro 2014]; WAO [Simons 2011]; WAO [Simons 2015]).
Oral: 1 mg/kg (maximum: 50 mg) as a single dose (Campbell 2014).
Angioedema (acute allergic) and/or urticaria (acute): Note: For moderate to severe symptoms without signs of anaphylaxis. Use epinephrine if anaphylaxis symptoms (eg, risk of airway or cardiovascular compromise) are present (Cicardi 2014; Zuraw 2019). In patients with acute urticaria, consider reserving use for patients with significant angioedema or whose symptoms are unresponsive to antihistamines (Asero 2019; Bernstein 2014; EAACI [Zuberbier 2018]; Powell 2015). The optimal dosing strategy has not been defined (Bernstein 2014; EAACI [Zuberbier 2018]; James 2017; Powell 2015).
Oral: Initial: 20 to 40 mg daily in 1 to 2 divided doses for 3 to 4 days (EAACI [Zuberbier 2018]; Powell 2015; Zuraw 2019). May consider tapering the dose for a total treatment duration of ≤10 days (EAACI [Zuberbier 2018]; Zuraw 2019).
Asthma, acute exacerbation: Note: For moderate to severe exacerbations or in patients who do not respond promptly and completely to short-acting beta agonists; administer within 1 hour of presentation to emergency department (GINA 2018; NAEPP 2007).
Oral: 40 to 60 mg daily for 3 to 10 days; administer in 1 or 2 divided doses. If symptoms do not resolve and peak expiratory flow is not at least 70% of personal best, then longer treatment may be required (GINA 2018; NAEPP 2007).
Bell palsy, new onset (off-label use): Oral: 60 to 80 mg daily for 5 to 7 days; administer in 1 or 2 divided doses; may be followed by a 5-day taper. Treatment should begin within 72 hours of onset of symptoms; a concomitant antiviral agent may be indicated in select patients (Austin 1993; de Almeida 2014; Engström 2008; OHNS [Baugh 2013]; Ronthal 2019).
Chronic obstructive pulmonary disease, acute exacerbation (off-label use): Note: In patients with severe but not life-threatening exacerbations, oral regimens are recommended. In patients who cannot tolerate oral therapy (eg, shock, mechanically ventilated), use IV methylprednisolone (GOLD 2019; Stoller 2019).
Oral: 40 to 60 mg once daily for 5 to 14 days (GOLD 2019; Stoller 2019). Note: The optimal dose has not been established. If patient improves with therapy, may discontinue without taper. If patient does not improve, a longer duration of therapy may be indicated (Stoller 2019).
Chronic spontaneous urticaria, acute exacerbation (off-label use): Note: For the temporary control of severe exacerbations (Bernstein 2014; EAACI [Zuberbier 2018]; Powell 2015).
Oral: 35 to 40 mg once daily until symptoms are controlled (usually occurs after 2 to 3 days of therapy) (Bernstein 2014; Khan 2019; Powell 2015); then taper by 5 to 10 mg/day over a period of 2 to 3 weeks followed by discontinuation (Khan 2019).
Duchenne muscular dystrophy (off-label use): Oral: 0.75 mg/kg/day (AAN [Gloss 2016]; Escolar 2011). Some experts use a maximum dose of 40 mg/day due to potential for greater adverse effects and decreased benefit at higher doses (Darras 2019).
Note: In patients who experience intolerable adverse effects, may decrease the dose by 25% to 33% (Birnkrant 2018). If adverse effects persist, continue to gradually taper to as low as 0.3 mg/kg/day, which may provide benefit (AAN [Gloss 2016]; Darras 2019). Doses as high as 1.5 mg/kg/day have been studied, but there is no evidence that doses >0.75 mg/kg/day provide greater efficacy (AAN [Gloss 2016]; Matthews 2016).
Giant cell arteritis, treatment (off-label use): Note: To reduce the risk of visual loss, start treatment immediately once diagnosis is highly suspected (BSR/BHPR [Dasgupta 2010]; Loddenkemper 2007). In patients presenting with threatened/evolving vision loss, pulse IV methylprednisolone is suggested as initial therapy prior to an oral glucocorticoid (eg, prednisone) (Docken 2019a).
Initial therapy in patients presenting without vision loss (or following initial therapy with pulse IV methylprednisolone in patients with threatened/evolving vision loss): Oral: High-dose: 1 mg/kg (maximum: 60 mg/day) once daily for 2 to 4 weeks; once signs/symptoms have declined and laboratory values have returned to normal or near normal, begin to taper until discontinuation over the next 6 to 12 months (BSR/BHPR [Dasgupta 2010]; Docken 2019a; González-Gay 2018).
Gout, acute flare: Oral: 0.5 mg/kg/day for 5 to 10 days followed by discontinuation (ACR [Khanna 2012]) or 30 to 40 mg/day given once daily or in 2 divided doses until symptom improvement, followed by a 7- to 10-day taper (or 14- to 21-day taper in patients with multiple prior flares) (Becker 2019). If unable to take orally, consider intra-articular or parenteral methylprednisolone.
Graft-versus-host disease, acute, treatment (off-label use): Note: For grade II or higher acute graft-versus-host disease. An optimal regimen has not been identified; refer to institutional protocols as variations exist. Treatment is dependent on the severity and the rate of progression (Martin 2012; Ruutu 2014).
Oral: Initial: 2 to 2.5 mg/kg/day in divided doses; dose may vary based on organ involvement and severity. Continue for several weeks then taper over several months (Chao 2019; Martin 2012).
Hepatitis, autoimmune (off-label use): Note: Approach to treatment should be patient specific and guided by response to treatment. Monotherapy induction regimen included below; other induction regimens (eg, combination therapy with a glucocorticoid-sparing agent) may be used in select patients (AASLD [Manns 2010]; EASL 2015; Heneghan 2019).
Induction: Initial: Oral: 60 mg once daily for 1 week, followed by a taper (eg, reduce dose by 10 mg/day at weekly intervals) based on symptoms and laboratory values until 20 mg once daily is reached for maintenance of remission (AASLD [Manns 2010]). Some experts initiate therapy at 40 mg once daily in select patients (Heneghan 2019).
Maintenance: Further taper dose to one that maintains remission (eg, taper the dose by 2.5 to 5 mg/day at weekly intervals to reach 5 mg/day). Specific maintenance approach will depend on patient response to initial treatment and tapering (AASLD [Manns 2010]).
Immune thrombocytopenia: Note: Goal of therapy is to provide a safe platelet count to prevent clinically important bleeding rather than normalization of the platelet count (Arnold 2019).
Initial therapy: Oral: 1 mg/kg/day for 1 to 2 weeks, followed by a gradual taper (Arnold 2019) or 0.5 to 2 mg/kg/day for several days to several weeks until platelet count increases (Provan 2010).
Pregnancy associated: Oral: Initial: 10 to 20 mg once daily (ACOG 207 2019). Adjust to the minimum effective dose to achieve response; generally, continue for at least 21 days, then taper to the minimum effective dose required to maintain platelet count to prevent major bleeding (ACOG 207 2019; ASH [Neunert 2011]) or 1 mg/kg/day for 2 weeks, followed by a gradual taper (George 2019).
Fetal alloimmune thrombocytopenia (maternal administration): Oral: 0.5 to 1 mg/kg/day. Dose is dependent upon gestational age and risk of fetal/neonatal intracranial hemorrhage and is administered in addition to immune globulin IV (ACOG 207 2019; Pacheco 2011).
Inflammatory bowel disease:
Crohn disease (moderate to severe or select patients with mild disease), induction: Note: Not for long-term use (ACG [Lichtenstein 2018]).
Oral: 40 to 60 mg once daily for 7 to 14 days, followed by a taper of up to 3 months (eg, reduce dose by 5 mg/day at weekly intervals until 20 mg/day is reached, then further reduce by 2.5 to 5 mg/day at weekly intervals) (ACG [Lichtenstein 2018]). Tapering regimens vary; some experts recommend a more rapid taper with a goal of discontinuing therapy within 1 to 2 months; if symptoms return, may resume therapy and taper more slowly (Regueiro 2019). Steroid-sparing agents (eg, biologic agents, immunomodulators) should be introduced with a goal of discontinuing corticosteroid therapy as soon as possible (ACG [Lichtenstein 2018]).
Ulcerative colitis (moderate to severe), induction: Note: Not for long-term use (ACG [Rubin 2019]).
Oral: 40 to 60 mg/day in 1 to 2 divided doses. Clinical improvement is expected within 7 days; pace of tapering (usually over 1 to 3 months) should be guided by symptoms, cumulative steroid exposure, and onset of action of additional therapies (ACG [Rubin 2019]; al Hashash 2019; Cohen 2019b).
Iodinated contrast media allergic-like reaction, prevention: Note: Generally reserved for patients with a prior allergic-like or unknown-type iodinated contrast reaction who will be receiving another iodinated contrast agent. Nonurgent premedication with an oral corticosteroid is generally preferred when contrast administration is scheduled to begin in ≥12 hours; however, consider an urgent (accelerated) regimen with an IV corticosteroid (eg, methylprednisolone) for those requiring contrast in <12 hours (ACR 2018).
Nonurgent regimen: Oral: 50 mg administered 13 hours, 7 hours, and 1 hour before contrast medium administration in combination with oral diphenhydramine 50 mg (administered 1 hour prior to contrast) (ACR 2018).
Minimal change disease, treatment (off-label use): Initial therapy: Oral: 1 mg/kg/day (maximum: 80 mg/day) once daily or 2 mg/kg every other day (maximum: 120 mg every other day) for 8 to 16 weeks (most patients experience remission by 16 weeks); after achieving remission, gradually taper (eg, decrease by 5 to 10 mg/week over a period of up to 6 months); the duration of initial pulse therapy and tapering schedule can vary (Hogan 2013; Meyrier 2019; Vivarelli 2017).
Multiple myeloma (previously untreated; transplant ineligible) (off-label use):
≥65 years of age or <65 years of age and transplant ineligible: Oral: 60 mg/m2/day for 4 days (days 1 to 4) every 6 weeks for 9 cycles (dexamethasone at a dose of 20 mg was substituted for prednisone on day 1 of each cycle) in combination with daratumumab, bortezomib, and melphalan; after cycle 9, daratumumab is continued as a single agent (Mateos 2018) or 60 mg/m2/day for 4 days (days 1 to 4) every 6 weeks (in combination with bortezomib and melphalan) for 9 cycles (San Miguel 2008) or 2 mg/kg/day for 4 days (days 1 to 4) every 6 weeks (in combination with melphalan and thalidomide) for 12 cycles (Facon 2007).
≥65 years of age: Oral: 2 mg/kg/day for 4 days (days 1 to 4) every 6 weeks (in combination with melphalan) for 12 cycles (Facon 2006).
Multiple sclerosis, acute exacerbation: Note: For patients with an acute exacerbation resulting in neurologic symptoms and increased disability or impairments in vision, strength, or cerebellar function (Olek 2019).
Initial pulse therapy using an oral glucocorticoid (alternative agent to IV methylprednisolone pulse therapy): Oral: 625 mg to 1.25 g daily for 3 to 7 days (5 days typically), either alone or followed by a taper (Morrow 2004; Olek 2019).
Taper following IV methylprednisolone or prednisone pulse therapy: Oral: 1 mg/kg/day (maximum: 80 mg/day), followed by a taper; total duration of oral therapy is usually 11 to 14 days (Goodin 2014; Murray 2006; Myhr 2009). Tapering schedules vary and some experts prefer to omit taper following initial pulse glucocorticoid therapy (Olek 2019).
Myasthenia gravis, crisis (adjunctive therapy) (off-label use): Note: Used in conjunction with immune globulin IV or plasma exchange (Bird 2019).
Oral: 1 mg/kg/day (usual dose range: 60 to 80 mg daily), followed by a taper (Bird 2019; Lacomis 2005).
Myopathies (dermatomyositis/polymyositis), treatment:
Initial therapy (or following initial therapy with pulse IV methylprednisolone in select patients): Oral: 1 mg/kg/day (maximum: 80 mg/day) as a single daily dose until improvement (usually for 4 to 6 weeks); then gradually tapered (total duration usually 9 to 12 months) (Dalakas 2011; Findlay 2015; Miller 2019). Note: Continuing high dose (1 mg/kg/day) for more than 6 weeks may increase risk of developing glucocorticoid-associated myopathy (Miller 2019).
Pericarditis, acute (alternative agent) (off-label use): Note: May be used for patients with contraindications (eg, renal failure) or incomplete response to aspirin/nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine (ESC [Adler 2015]). Glucocorticoid therapy early in the course of pericarditis is more likely to be associated with recurrent episodes (Imazio 2020).
Oral: 0.2 to 0.5 mg/kg/day until resolution of symptoms and normalization of CRP (2 to 4 weeks); then taper dose over 3 months (ESC [Adler 2015]; Imazio 2020).
Pneumocystis pneumonia, adjunctive therapy for moderate to severe disease (off-label use): Note: Recommended when on room air PaO2 <70 mm Hg or PAO2-PaO2 ≥35 mm Hg.
Oral: 40 mg twice daily on days 1 to 5 beginning as early as possible, followed by 40 mg once daily on days 6 to 10, then 20 mg once daily on days 11 to 21 (HHS [OI adult 2019]; Martin 2013; Sax 2019; Thomas 2019).
Polymyalgia rheumatica: Note: Goal of therapy is to alleviate symptoms; therapy has not been shown to improve prognosis or prevent progression to giant cell arteritis (Docken 2019b).
Oral: Initial: Usual dose: 15 mg/day in a single daily dose or in divided doses; some experts consider lower initial doses of 7.5 to 10 mg/day for smaller patients with mild symptoms or at high risk for side effects (eg, labile diabetes) and higher initial doses of 20 mg/day (or 25 mg/daily [rarely]) for patients with more severe symptoms. Divided doses may help with pain and stiffness in evenings and following morning. Once symptoms are controlled, maintain dose for 2 to 4 weeks and gradually taper (generally over a 1- to 2-year period); some patients may require longer treatment (Castañeda 2019; EULAR/ACR [Dejaco 2015]; Docken 2019b).
Prostate cancer, metastatic, castration resistant (off-label use): Oral: 5 mg twice daily (in combination with abiraterone) until disease progression or unacceptable toxicity (de Bono 2011; Ryan 2015) or 10 mg once daily (in combination with cabazitaxel) for up to 10 cycles (de Bono 2010) or 5 mg twice daily (in combination with docetaxel) for up to 10 cycles (Berthold 2008; Tannock 2004).
Systemic rheumatic disorders (eg, antineutrophil cytoplasmic antibody-associated vasculitis, mixed cryoglobulinemia syndrome, polyarteritis nodosa, rheumatoid arthritis, systemic lupus erythematosus): Note: The following dosage ranges are for guidance only; dosing should be highly individualized, taking into account disease severity, the specific disorder, and disease manifestations:
Mild to moderate disease: Oral: Initial: 5 to 30 mg/day in a single daily dose or in divided doses, then taper to the minimum effective dose, depending on response (ACR 2002; Cohen 2019a; Fervenza 2019; O’Dell 2019; Wallace 2019).
Severe disease: Initial therapy (or following initial therapy with pulse IV methylprednisolone in select patients):
Oral: Usual dose: Initial: 1 mg/kg/day (maximum: 60 to 80 mg/day) in a single daily dose or in divided doses; typically given for several weeks, then tapered gradually; may be given as part of an appropriate combination regimen; for severe systemic lupus erythematosus, up to 2 mg/kg/day may be given initially (Merkel 2019b; Fervenza 2019; Muchtar 2017; Pietrogrande 2011; Wallace 2019).
Takayasu arteritis (off-label use): Oral: Initial: 40 to 60 mg daily in combination with appropriate steroid-sparing agent; gradually taper to lowest effective dose (ACCF/AHA [Hiratzka 2010]; EULAR [Hellmich 2020]); some experts initiate treatment with 1 mg/kg/day (maximum: 60 to 80 mg/day) (Merkel 2019a). Note: Long-term therapy may be required to prevent progression (ACCF/AHA [Hiratzka 2010]; Merkel 2019a).
Thyroiditis, subacute (off-label use): Note: For use in patients whose pain does not respond to full dose of NSAIDs over several days or patients who present initially with moderate to severe pain (ATA [Ross 2016]).
Oral: Initial: 40 mg/day for 1 to 2 weeks; gradually taper (eg, by 5 to 10 mg/day every 5 to 7 days) based on clinical response. If pain recurs, increase to the lowest dose that controlled the pain; maintain that dose for ~2 weeks and attempt to taper again (ATA [Ross 2016]; Burman 2019).
Tuberculosis, pulmonary (prevention of immune reconstitution inflammatory syndrome in HIV-infected patients) (off-label use): Note: For use in antiretroviral-naive patients with CD4 count ≤100 cells/mm3 who start antiretroviral therapy within 30 days of antituberculosis therapy initiation (Meintjes 2018).
Oral: 40 mg once daily for 14 days, followed by 20 mg once daily for 14 days, during the first 4 weeks after initiation of antiretroviral therapy (Meintjes 2018).
Warm autoimmune hemolytic anemia: Oral: 1 to 2 mg/kg/day until a hemoglobin response has occurred (typically within 1 to 3 weeks). After hemoglobin stabilization, begin tapering to the lowest dose to maintain remission followed by gradual tapering with an eventual goal of discontinuation (total duration of therapy: 3 to 12 months); a clinician experienced with the treatment of hemolytic anemia should be involved with therapy (Barros 2010; Brodsky 2019; Roumier 2014; Zanella 2014).
Dosing: Geriatric
Refer to adult dosing; use the lowest effective dose.
Dosing: Pediatric
Note: All pediatric dosing based on immediate release products. Dose depends upon condition being treated and response of patient; dosage for infants and children should be based on disease severity and patient response rather than by rigid adherence to dosage guidelines by age, weight, or body surface area. Consider alternate day therapy for long-term therapy. Discontinuation of long-term therapy requires gradual withdrawal by tapering the dose.
General dosing; anti-inflammatory or immunosuppressive: Infants, Children, and Adolescents: Oral 0.05 to 2 mg/kg/day divided every 6 to 24 hours (Bertsias 2012; Kliegman 2007)
Asthma, acute exacerbation:
NAEPP 2007:
Infants and Children <12 years: Oral:
Emergency care or hospital doses: 1 to 2 mg/kg/day in 2 divided doses; maximum daily dose: 60 mg/day; continue until peak expiratory flow is 70% of predicted or personal best
Short-course “burst” (Outpatient): 1 to 2 mg/kg/day in divided doses 1 to 2 times daily for 3 to 10 days; maximum daily dose: 60 mg/day; Note: Burst should be continued until symptoms resolve or patient achieves peak expiratory flow 80% of personal best; usually requires 3 to 10 days of treatment (~5 days on average); longer treatment may be required
Children ≥12 years and Adolescents: Oral:
Emergency care or hospital doses: 40 to 80 mg/day in divided doses 1 to 2 times daily until peak expiratory flow is 70% of predicted or personal best
Short-course “burst” (Outpatient): 40 to 60 mg/day in divided doses 1 to 2 times daily for 3 to 10 days; Note: Burst should be continued until symptoms resolve and peak expiratory flow is at least 80% of personal best; usually requires 3 to 10 days of treatment (~5 days on average); longer treatment may be required
GINA 2014:
Infants and Children <12 years: Oral: 1 to 2 mg/kg/day for 3 to 5 days
Maximum daily dose age-dependent:
Infants and Children <2 years: 20 mg/day
Children 2 to 5 years: 30 mg/day
Children 6 to 11 years: 40 mg/day
Children ≥12 years and Adolescents: Oral: 1 mg/kg/day for 5 to 7 days; maximum daily dose 50 mg/day
Asthma, maintenance therapy (nonacute) (NAEPP 2007):
Infants and Children <12 year: Oral: 0.25 to 2 mg/kg/day administered as a single dose in the morning or every other day as needed for asthma control; maximum daily dose: 60 mg/day
Children ≥12 years and Adolescents: Oral: 7.5 to 60 mg daily administered as a single dose in the morning or every other day as needed for asthma control
Autoimmune hepatitis (monotherapy or in combination with azathioprine): Limited data available: Infants, Children, and Adolescents: Oral: Initial: 1 to 2 mg/kg/day for 2 weeks; maximum daily dose: 60 mg/day; taper upon response over 6 to 8 weeks to a dose of 0.1 to 0.2 mg/kg/day or 2.5 to 5 mg daily; an alternate day schedule to decrease risk of adverse effect has been used; however, a higher incidence of relapse has been observed in some cases and use is not suggested (AASLD [Manns 2010]; Della Corte 2012).
Bell palsy: Limited data available:
Infants, Children, and Adolescents <16 years: Oral: 1 mg/kg/day for 1 week, then taper over 1 week; ideally start within the 72 hours of onset of symptoms; maximum daily dose: 60 mg/day (Kliegman 2011)
Adolescents ≥16 years: Oral: 60 mg daily for 5 days, followed by a 5-day taper. Treatment should begin within 72 hours of onset of symptoms (OHNS [Baugh 2013]).
Congenital adrenal hyperplasia: Note: Individualize dose by monitoring growth, hormone levels, and bone age; mineralocorticoid (eg, fludrocortisone) and sodium supplement may be required in salt losers (AAP 2000; Endocrine Society [Speiser] 2010):
Infants, Children and Adolescents (actively growing): Not recommended because impedes statural growth more so than shorter-acting systemic glucocorticoid (ie, hydrocortisone) (Endocrine Society [Speiser] 2010)
Adolescents (fully grown): Oral: 5 to 7.5 mg daily in divided doses 2 times daily (Endocrine Society [Speiser] 2010)
Crohn disease: Children and Adolescents: Oral: 1 to 2 mg/kg/day; maximum daily dose: 60 mg/day; continue for 2 to 4 weeks until remission, then gradually taper (Kliegman 2011; Rufo 2012; Sandhu 2010)
Dermatomyositis, moderately severe; initial treatment: Limited data available: Children and Adolescents: Oral: Initial: 2 mg/kg/day divided once or twice daily; maximum daily dose: 60 mg/day; continue for 4 weeks then if adequate patient response, begin taper; most recommend an initial 20% reduction in dose with subsequent wean based upon response; use in combination with other immunosuppressants (eg, methotrexate) (CARRA [Huber 2010])
Immune thrombocytopenia (ITP): Infants, Children, and Adolescents: Oral: 1 to 2 mg/kg/day; titrate dose according to platelet count; when and if able, a rapid taper is recommended; a maximum duration of therapy of 14 days has been suggested; others have used a higher dose with shorter course of 4 mg/kg/day for 3 to 4 days (Neunert 2011; Provan 2010)
Juvenile idiopathic arthritis: Infants ≥ 6 months, Children and Adolescents: Oral: Initial: 1 mg/kg/day administered once daily (maximum daily dose: 60 mg/day); may be used in combination with methylprednisolone pulse therapy; evaluate initial response at 1 to 2 weeks and then at 1 month of therapy; if patient improves then taper prednisone, if unchanged then continue current prednisone therapy and if worsened then increase dose to 2 mg/kg/day (maximum daily dose: 100 mg/day) . After 1 month, if improvement, begin taper; if condition worsens or unchanged then increase or continue prednisone dose at 2 mg/kg/day (maximum daily dose: 100 mg/day) and/or may add or repeat methylprednisolone pulse therapy. After 3 months of glucocorticoid therapy, if improvement (prednisone dose <50% starting dose), continue taper and reassess monthly; if patient remains unchanged (prednisone dose >50% of starting dose) or worsened, additional therapy should be considered (CARRA [Dewitt 2012])
Lupus nephritis: Children and Adolescents: Oral: Initial therapy:
With concurrent methylprednisolone pulse therapy: Prednisone: 0.5 to 1.5 mg/kg/day; maximum daily dose: 60 mg/day, taper usually over 6 months to a dose ≤10 mg/day according to clinical response; use in combination with cyclophosphamide or mycophenolate (Bertsias 2012; KDIGO 2012; KDOQI 2013; Mina 2012)
Without concurrent methylprednisolone pulse therapy: Prednisone: 2 mg/kg/day for 6 weeks, maximum daily dose variable: for weeks 1 to 4: maximum daily dose: 80 mg/day and for weeks 5 and 6: 60 mg/day; taper over 6 months; use in combination with cyclophosphamide or mycophenolate (Mina 2012)
Malignancy (antineoplastic): Note: Used for various types of malignancy and neoplasm; see specific protocols for details concerning dosing in combination regimens.
Hodgkin lymphoma (BEACOPP regimen): Children and Adolescents: Oral: 40 mg/m2/day in 2 divided doses on days 0 to 13; in combination with bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, and procarbazine (Kelly 2002; Kelly 2011)
Nephrotic syndrome; steroid-sensitive (SSNS): Children and Adolescents: Note: Obese patients should be dosed based on ideal body weight: Oral:
Initial episode: 2 mg/kg/day or 60 mg/m2/day once daily, maximum daily dose: 60 mg/day for 4 to 6 weeks; then adjust to an alternate-day schedule of 1.5 mg/kg/dose or 40 mg/m2/dose on alternate days as a single dose, maximum dose: 40 mg/dose (Gipson 2009; KDIGO 2012; KDOQI 2013); duration of therapy based on patient response.
Relapse: 2 mg/kg/day or 60 mg/m2/day once daily, maximum daily dose: 60 mg/day continue until complete remission for at least 3 days; then adjust to an alternate-day schedule of 1.5 mg/kg/dose or 40 mg/m2/dose on alternate days as a single dose, maximum dose: 40 mg/dose, recommended duration of alternate day dosing is variable: may continue for at least 4 weeks then taper. Longer duration of treatment may be necessary in patients who relapse frequently, some patients may require up to 3 months of treatment (Gipson 2009; KDIGO 2012; KDOQI 2013).
Maintenance therapy for frequently relapsing SSNS: Taper previous dose down to lowest effective dose which maintains remission using an alternate day schedule; usual effective range: 0.1 to 0.5 mg/kg/dose on alternating days; other patients may require doses up to 0.7 mg/kg/dose every other day (KDIGO 2012, KDOQI 2013)
Physiologic replacement: Children and Adolescents: Oral: 2 to 2.5 mg/m2/day (Ahmet 2011; Gupta 2008). Note: Hydrocortisone is generally preferred in growing children and adolescents due to its lower growth suppressant effects compared to prednisone (Gupta 2008).
Pneumocystis jirovecii pneumonia (PCP), treatment; HIV-exposed/-positive: Note: Begin as soon as possible after diagnosis and within 72 hours of PCP therapy initiation.
Infants and Children: Oral: 1 mg/kg/dose twice daily on days 1 to 5, then 0.5 to 1 mg/kg/dose twice daily on days 6 to 10, then 0.5 mg/kg/dose once daily for days 11 to 21 (DHHS [pediatric] 2013)
Adolescents: Oral: 40 mg twice daily on days 1 to 5, followed by 40 mg once daily on days 6 to 10, followed by 20 mg once daily on days 11 to 21 or until antimicrobial regimen is completed (DHHS [adult] 2014).
Ulcerative colitis: Children and Adolescents: Oral: 1 to 2 mg/kg/day administered in the morning; maximum daily dose: 60 mg/day; if no response after 7 to 14 days optimal dosing and compliance should be assessed (Kliegman 2011; Rufo 2012; Turner 2012)
Administration
Administer after meals or with food or milk to decrease GI upset. May administer antacids between meals to help prevent peptic ulcers.
Delayed-release tablets: Swallow whole; do not break, divide, crush, or chew.
Oral solution, concentrate: Administer only with provided calibrated dropper.
Dietary Considerations
May require increased dietary intake of pyridoxine, vitamin C, vitamin D, folate, calcium, and phosphorus; may require decreased dietary intake of sodium and potassium supplementation
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Protect from light and moisture.
Oral solution, concentrate: Discard opened bottle after 90 days.
PredniSONE Images
Drug Interactions
Acetylcholinesterase Inhibitors: Corticosteroids (Systemic) may enhance the adverse/toxic effect of Acetylcholinesterase Inhibitors. Increased muscular weakness may occur. Monitor therapy
Aldesleukin: Corticosteroids may diminish the antineoplastic effect of Aldesleukin. Avoid combination
Amphotericin B: Corticosteroids (Systemic) may enhance the hypokalemic effect of Amphotericin B. Monitor therapy
Androgens: Corticosteroids (Systemic) may enhance the fluid-retaining effect of Androgens. Monitor therapy
Antacids: May decrease the bioavailability of Corticosteroids (Oral). Management: Consider separating doses by 2 or more hours. Budesonide enteric coated tablets could dissolve prematurely if given with drugs that lower gastric acid, with unknown impact on budesonide therapeutic effects. Consider therapy modification
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Aprepitant: May increase the serum concentration of Corticosteroids (Systemic). Management: No dose adjustment is needed for single 40 mg aprepitant doses. For other regimens, reduce oral dexamethasone or methylprednisolone doses by 50%, and IV methylprednisolone doses by 25%. Antiemetic regimens containing dexamethasone reflect this adjustment. Consider therapy modification
Axicabtagene Ciloleucel: Corticosteroids (Systemic) may diminish the therapeutic effect of Axicabtagene Ciloleucel. Management: Avoid use of corticosteroids as premedication before axicabtagene ciloleucel. Corticosteroids may, however, be required for treatment of cytokine release syndrome or neurologic toxicity. Consider therapy modification
Baricitinib: Immunosuppressants may enhance the immunosuppressive effect of Baricitinib. Management: Use of baricitinib in combination with potent immunosuppressants such as azathioprine or cyclosporine is not recommended. Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted. Consider therapy modification
BCG (Intravesical): Immunosuppressants may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Bile Acid Sequestrants: May decrease the absorption of Corticosteroids (Oral). Monitor therapy
Calcitriol (Systemic): Corticosteroids (Systemic) may diminish the therapeutic effect of Calcitriol (Systemic). Monitor therapy
Cladribine: May enhance the immunosuppressive effect of Immunosuppressants. Avoid combination
Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Monitor therapy
Corticorelin: Corticosteroids may diminish the therapeutic effect of Corticorelin. Specifically, the plasma ACTH response to corticorelin may be blunted by recent or current corticosteroid therapy. Monitor therapy
Cosyntropin: Corticosteroids (Systemic) may diminish the diagnostic effect of Cosyntropin. Monitor therapy
CycloSPORINE (Systemic): May increase serum concentrations of the active metabolite(s) of PredniSONE. PredniSONE may decrease the serum concentration of CycloSPORINE (Systemic). PredniSONE may increase the serum concentration of CycloSPORINE (Systemic). Monitor therapy
CYP3A4 Inducers (Strong): May decrease the serum concentration of PredniSONE. Monitor therapy
CYP3A4 Inhibitors (Strong): May increase the serum concentration of PredniSONE. Monitor therapy
Deferasirox: Corticosteroids (Systemic) may enhance the adverse/toxic effect of Deferasirox. Specifically, the risk for GI ulceration/irritation or GI bleeding may be increased. Monitor therapy
Deferasirox: Corticosteroids may enhance the adverse/toxic effect of Deferasirox. Specifically, the risk for GI ulceration/irritation or GI bleeding may be increased. Monitor therapy
Denosumab: May enhance the adverse/toxic effect of Immunosuppressants. Specifically, the risk for serious infections may be increased. Monitor therapy
Desirudin: Corticosteroids (Systemic) may enhance the anticoagulant effect of Desirudin. More specifically, corticosteroids may increase hemorrhagic risk during desirudin treatment. Management: Discontinue treatment with systemic corticosteroids prior to desirudin initiation. If concomitant use cannot be avoided, monitor patients receiving these combinations closely for clinical and laboratory evidence of excessive anticoagulation. Consider therapy modification
Desmopressin: Corticosteroids (Systemic) may enhance the hyponatremic effect of Desmopressin. Avoid combination
DilTIAZem: May increase the serum concentration of Corticosteroids (Systemic). Monitor therapy
Echinacea: May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification
Estrogen Derivatives: May increase the serum concentration of Corticosteroids (Systemic). Monitor therapy
Fexinidazole [INT]: Corticosteroids (Systemic) may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Fingolimod: Immunosuppressants may enhance the immunosuppressive effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and other immunosuppressants when possible. If combined, monitor patients closely for additive immunosuppressant effects (eg, infections). Consider therapy modification
Fluconazole: May increase the serum concentration of PredniSONE. Monitor therapy
Fosaprepitant: May increase the serum concentration of Corticosteroids (Systemic). The active metabolite aprepitant is likely responsible for this effect. Consider therapy modification
Hyaluronidase: Corticosteroids may diminish the therapeutic effect of Hyaluronidase. Management: Patients receiving corticosteroids (particularly at larger doses) may not experience the desired clinical response to standard doses of hyaluronidase. Larger doses of hyaluronidase may be required. Consider therapy modification
Indacaterol: May enhance the hypokalemic effect of Corticosteroids (Systemic). Monitor therapy
Indium 111 Capromab Pendetide: Corticosteroids (Systemic) may diminish the diagnostic effect of Indium 111 Capromab Pendetide. Avoid combination
Isoniazid: Corticosteroids (Systemic) may decrease the serum concentration of Isoniazid. Monitor therapy
Leflunomide: Immunosuppressants may enhance the adverse/toxic effect of Leflunomide. Specifically, the risk for hematologic toxicity such as pancytopenia, agranulocytosis, and/or thrombocytopenia may be increased. Management: Consider not using a leflunomide loading dose in patients receiving other immunosuppressants. Patients receiving both leflunomide and another immunosuppressant should be monitored for bone marrow suppression at least monthly. Consider therapy modification
Loop Diuretics: Corticosteroids (Systemic) may enhance the hypokalemic effect of Loop Diuretics. Monitor therapy
Macimorelin: Corticosteroids (Systemic) may diminish the diagnostic effect of Macimorelin. Avoid combination
Mifamurtide: Corticosteroids (Systemic) may diminish the therapeutic effect of Mifamurtide. Avoid combination
MiFEPRIStone: May diminish the therapeutic effect of Corticosteroids (Systemic). MiFEPRIStone may increase the serum concentration of Corticosteroids (Systemic). Management: Avoid mifepristone in patients who require long-term corticosteroid treatment of serious illnesses or conditions (e.g., for immunosuppression following transplantation). Corticosteroid effects may be reduced by mifepristone treatment. Avoid combination
Mitotane: May decrease the serum concentration of Corticosteroids (Systemic). Consider therapy modification
Natalizumab: Immunosuppressants may enhance the adverse/toxic effect of Natalizumab. Specifically, the risk of concurrent infection may be increased. Avoid combination
Neuromuscular-Blocking Agents (Nondepolarizing): May enhance the adverse neuromuscular effect of Corticosteroids (Systemic). Increased muscle weakness, possibly progressing to polyneuropathies and myopathies, may occur. Consider therapy modification
Nicorandil: Corticosteroids (Systemic) may enhance the adverse/toxic effect of Nicorandil. Gastrointestinal perforation has been reported in association with this combination. Monitor therapy
Nivolumab: Immunosuppressants may diminish the therapeutic effect of Nivolumab. Consider therapy modification
Nonsteroidal Anti-Inflammatory Agents (COX-2 Selective): Corticosteroids (Systemic) may enhance the adverse/toxic effect of Nonsteroidal Anti-Inflammatory Agents (COX-2 Selective). Monitor therapy
Nonsteroidal Anti-Inflammatory Agents (Nonselective): Corticosteroids (Systemic) may enhance the adverse/toxic effect of Nonsteroidal Anti-Inflammatory Agents (Nonselective). Monitor therapy
Ocrelizumab: May enhance the immunosuppressive effect of Immunosuppressants. Monitor therapy
Pidotimod: Immunosuppressants may diminish the therapeutic effect of Pidotimod. Monitor therapy
Pimecrolimus: May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Quinolones: Corticosteroids (Systemic) may enhance the adverse/toxic effect of Quinolones. Specifically, the risk of tendonitis and tendon rupture may be increased. Monitor therapy
Ritodrine: Corticosteroids may enhance the adverse/toxic effect of Ritodrine. Monitor therapy
Ritonavir: May increase the serum concentration of PredniSONE. Monitor therapy
Roflumilast: May enhance the immunosuppressive effect of Immunosuppressants. Consider therapy modification
Salicylates: May enhance the adverse/toxic effect of Corticosteroids (Systemic). These specifically include gastrointestinal ulceration and bleeding. Corticosteroids (Systemic) may decrease the serum concentration of Salicylates. Withdrawal of corticosteroids may result in salicylate toxicity. Monitor therapy
Sargramostim: Corticosteroids (Systemic) may enhance the therapeutic effect of Sargramostim. Specifically, corticosteroids may enhance the myeloproliferative effects of sargramostim. Monitor therapy
Siponimod: Immunosuppressants may enhance the immunosuppressive effect of Siponimod. Monitor therapy
Sipuleucel-T: Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Management: Evaluate patients to see if it is medically appropriate to reduce or discontinue therapy with immunosuppressants prior to initiating sipuleucel-T therapy. Consider therapy modification
Somatropin: PredniSONE may enhance the therapeutic effect of Somatropin. Somatropin may diminish the therapeutic effect of PredniSONE. Growth hormone may reduce the conversion of prednisone to the active prednisolone metabolite. Monitor therapy
Tacrolimus (Systemic): Corticosteroids (Systemic) may decrease the serum concentration of Tacrolimus (Systemic). Conversely, when discontinuing corticosteroid therapy, tacrolimus concentrations may increase. Monitor therapy
Tacrolimus (Topical): May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide. Monitor therapy
Tesamorelin: May decrease serum concentrations of the active metabolite(s) of PredniSONE. Consider therapy modification
Thiazide and Thiazide-Like Diuretics: Corticosteroids (Systemic) may enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Tisagenlecleucel: Corticosteroids (Systemic) may diminish the therapeutic effect of Tisagenlecleucel. Management: Avoid use of corticosteroids as premedication or at any time during treatment with tisagenlecleucel, except in the case of life-threatening emergency (such as resistant cytokine release syndrome). Consider therapy modification
Tofacitinib: Immunosuppressants may enhance the immunosuppressive effect of Tofacitinib. Management: Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted, and this warning seems particularly focused on more potent immunosuppressants. Consider therapy modification
Trastuzumab: May enhance the neutropenic effect of Immunosuppressants. Monitor therapy
Upadacitinib: Immunosuppressants may enhance the immunosuppressive effect of Upadacitinib. Avoid combination
Urea Cycle Disorder Agents: Corticosteroids (Systemic) may diminish the therapeutic effect of Urea Cycle Disorder Agents. More specifically, Corticosteroids (Systemic) may increase protein catabolism and plasma ammonia concentrations, thereby increasing the doses of Urea Cycle Disorder Agents needed to maintain these concentrations in the target range. Monitor therapy
Vaccines (Inactivated): Immunosuppressants may diminish the therapeutic effect of Vaccines (Inactivated). Management: Vaccine efficacy may be reduced. Complete all age-appropriate vaccinations at least 2 weeks prior to starting an immunosuppressant. If vaccinated during immunosuppressant therapy, revaccinate at least 3 months after immunosuppressant discontinuation. Consider therapy modification
Vaccines (Live): Corticosteroids (Systemic) may enhance the adverse/toxic effect of Vaccines (Live). Corticosteroids (Systemic) may diminish the therapeutic effect of Vaccines (Live). Management: Doses equivalent to less than 2 mg/kg or 20 mg per day of prednisone administered for less than 2 weeks are not considered sufficiently immunosuppressive to create vaccine safety concerns. Higher doses and longer durations should be avoided. Consider therapy modification
Warfarin: Corticosteroids (Systemic) may enhance the anticoagulant effect of Warfarin. Monitor therapy
Test Interactions
Decreased response to skin tests
Adverse Reactions
Frequency not defined.
Cardiovascular: Cardiac failure (in susceptible patients), hypertension
Central nervous system: Emotional lability, headache, increased intracranial pressure (with papilledema), myasthenia, psychiatric disturbance (including euphoria, insomnia, mood swings, personality changes, severe depression), seizure, vertigo
Dermatologic: Diaphoresis, facial erythema, skin atrophy, urticaria
Endocrine & metabolic: Cushing’s syndrome, decreased serum potassium, diabetes mellitus, fluid retention, growth suppression (children), hypokalemic alkalosis, hypothyroidism (enhanced), menstrual disease, negative nitrogen balance (due to protein catabolism), sodium retention
Gastrointestinal: Abdominal distention, carbohydrate intolerance, pancreatitis, peptic ulcer (with possible perforation and hemorrhage), ulcerative esophagitis
Hematologic & oncologic: Bruise, Kaposi’s sarcoma, petechia
Hepatic: Increased serum alkaline phosphatase, increased serum ALT, increased serum AST
Hypersensitivity: Anaphylaxis, hypersensitivity reaction
Infection: Infection
Neuromuscular & skeletal: Amyotrophy, aseptic necrosis of bones (femoral and humeral heads), osteoporosis, pathological fracture (long bones), rupture of tendon (particularly Achilles tendon), steroid myopathy, vertebral compression fracture
Ophthalmic: Exophthalmos, glaucoma, increased intraocular pressure, subcapsular posterior cataract
Miscellaneous: Wound healing impairment
<1%, postmarketing, and/or case reports: Venous thrombosis (Johannesdottir 2013)
Warnings/Precautions
Concerns related to adverse effects:
- Adrenal suppression: May cause hypercortisolism or suppression of hypothalamic-pituitary-adrenal (HPA) axis, particularly in younger children or in patients receiving high doses for prolonged periods. HPA axis suppression may lead to adrenal crisis. Withdrawal and discontinuation of a corticosteroid should be done slowly and carefully. Particular care is required when patients are transferred from systemic corticosteroids to inhaled products due to possible adrenal insufficiency or withdrawal from steroids, including an increase in allergic symptoms. Adult patients receiving >20 mg per day of prednisone (or equivalent) may be most susceptible. Fatalities have occurred due to adrenal insufficiency in asthmatic patients during and after transfer from systemic corticosteroids to aerosol steroids; aerosol steroids do not provide the systemic steroid needed to treat patients having trauma, surgery, or infections.
- Anaphylactoid reactions: Rare cases of anaphylactoid reactions have been observed in patients receiving corticosteroids.
- Immunosuppression: Prolonged use of corticosteroids may increase the incidence of secondary infection, mask acute infection (including fungal infections), prolong or exacerbate viral infections, or limit response to killed or inactivated vaccines. Exposure to chickenpox or measles should be avoided. Corticosteroids should not be used to treat viral hepatitis or cerebral malaria. Close observation is required in patients with latent tuberculosis (TB) and/or TB reactivity; restrict use in active TB (only fulminating or disseminated TB in conjunction with antituberculosis treatment). Latent or active amebiasis should be ruled out in any patient with recent travel to tropic climates or unexplained diarrhea prior to corticosteroid initiation. Use with extreme caution in patients with Strongyloides infections; hyperinfection, dissemination and fatalities have occurred.
- Kaposi sarcoma: Prolonged treatment with corticosteroids has been associated with the development of Kaposi sarcoma (case reports); if noted, discontinuation of therapy should be considered (Goedert 2002).
- Myopathy: Acute myopathy has been reported with high dose corticosteroids, usually in patients with neuromuscular transmission disorders; may involve ocular and/or respiratory muscles; monitor creatine kinase; recovery may be delayed.
- Psychiatric disturbances: Corticosteroid use may cause psychiatric disturbances, including euphoria, insomnia, mood swings, personality changes, severe depression or frank psychotic manifestations. Preexisting psychiatric conditions may be exacerbated by corticosteroid use.
Disease-related concerns:
- Cardiovascular disease: Use with caution in patients with heart failure and/or hypertension; long-term use has been associated with electrolyte disturbances, fluid retention, and hypertension. Use with caution in patients with a recent history of myocardial infarction; left ventricular free wall rupture has been reported after the use of corticosteroids.
- Diabetes: Use corticosteroids with caution in patients with diabetes mellitus; may alter glucose production/regulation leading to hyperglycemia.
- Gastrointestinal disease: Use with caution in patients with GI diseases (diverticulitis, fresh intestinal anastomoses, active or latent peptic ulcer, ulcerative colitis [nonspecific]) due to perforation risk.
- Head injury: Increased mortality was observed in patients receiving high-dose IV methylprednisolone; high-dose corticosteroids should not be used for the management of head injury.
- Hepatic impairment: Use with caution in patients with hepatic impairment, including cirrhosis; effects may be enhanced.
- Myasthenia gravis: Use with caution in patients with myasthenia gravis; exacerbation of symptoms has occurred especially during initial treatment with corticosteroids.
- Ocular disease: Use with caution in patients with cataracts and/or glaucoma; increased intraocular pressure, open-angle glaucoma, and cataracts have occurred with prolonged use. Use with caution in patients with a history of ocular herpes simplex; corneal perforation has occurred; do not use in active ocular herpes simplex. Consider routine eye exams in chronic users.
- Osteoporosis: Use with caution in patients with or who are at risk for osteoporosis; high doses and/or long-term use of corticosteroids have been associated with increased bone loss and osteoporotic fractures.
- Renal impairment: Use with caution in patients with renal impairment; fluid retention may occur.
- Seizure disorders: Use corticosteroids with caution in patients with a history of seizure disorder; seizures have been reported with adrenal crisis.
- Systemic sclerosis (scleroderma): Use of higher-dose corticosteroid therapy (in adults, ≥15 mg/day of prednisone or equivalent) in patients with systemic sclerosis may increase the risk of scleroderma renal crisis; avoid use when possible (Steen 1998; Trang 2012).
- Thyroid disease: Changes in thyroid status may necessitate dosage adjustments; metabolic clearance of corticosteroids increases in hyperthyroid patients and decreases in hypothyroid patients.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Elderly: Use with caution in elderly patients with the smallest possible effective dose for the shortest duration.
- Pediatric: May affect growth velocity; growth and development should be routinely monitored in pediatric patients.
Dosage form specific issues:
- Benzyl alcohol and derivatives: Some dosage forms may contain sodium benzoate/benzoic acid; benzoic acid (benzoate) is a metabolite of benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors 2001); avoid or use dosage forms containing benzyl alcohol derivative with caution in neonates. See manufacturer's labeling.
- Propylene glycol: Some dosage forms may contain propylene glycol; large amounts are potentially toxic and have been associated hyperosmolality, lactic acidosis, seizures, and respiratory depression; use caution (AAP ["Inactive" 1997]; Zar 2007).
Other warnings/precautions:
- Discontinuation of therapy: Withdraw therapy with gradual tapering of dose.
- Stress: Patients may require higher doses when subject to stress (ie, trauma, surgery, severe infection).
Monitoring Parameters
Blood pressure; weight; serum glucose; electrolytes; growth in pediatric patients; presence of infection, bone mineral density; assess HPA axis suppression (eg, ACTH stimulation test, morning plasma cortisol test, urinary free cortisol test); Hgb, occult blood loss, leukopenia and thrombocytopenia (every 6 months when used in combination with azathioprine [Manns 2010]); chest x-ray (at regular intervals during prolonged therapy); IOP with therapy >6 weeks, eye examination (periodically during therapy [Manns 2010]).
Pregnancy
Pregnancy Considerations
Prednisone and its metabolite, prednisolone, cross the placenta.
In the mother, prednisone is converted to the active metabolite prednisolone by the liver. Prior to reaching the fetus, prednisolone is converted by placental enzymes back to prednisone. As a result, the level of prednisone remaining in the maternal serum and reaching the fetus are similar; however, the amount of prednisolone reaching the fetus is ~8 to 10 times lower than the maternal serum concentration (healthy women at term) (Beitins 1972).
Some studies have shown an association between first trimester systemic corticosteroid use and oral clefts or decreased birth weight; however, information is conflicting and may be influenced by maternal dose, duration/frequency of exposure, and indication for use (Lunghi 2010; Park-Wyllie 2000; Pradat 2003). Hypoadrenalism may occur in newborns following maternal use of corticosteroids in pregnancy; monitor. An increased risk of adverse maternal outcomes, including gestational diabetes, may be associated with use of high doses over extended periods (Murase 2014; Rademaker 2018).
When systemic corticosteroids are needed in pregnancy for rheumatic disorders, it is generally recommended to use the lowest effective dose for the shortest duration of time, avoiding high doses during the first trimester (Götestam Skorpen 2016; Makol 2011; Østensen 2009).
For dermatologic disorders in pregnant women, systemic corticosteroids are generally not preferred for initial therapy; should be avoided during the first trimester; and used during the second or third trimester at the lowest effective dose (Leachman 2006). Prednisone is preferred by some guidelines when an oral corticosteroid is needed because placental enzymes limit passage to the embryo (Murase 2014; Rademaker 2018).
Pregnant women with poorly controlled asthma or asthma exacerbations may have a greater fetal/maternal risk than what is associated with appropriately used medications. Uncontrolled asthma is associated with an increased risk of perinatal mortality, preeclampsia, preterm birth, and low birth weight infants. Inhaled corticosteroids are recommended for the treatment of asthma during pregnancy; however, systemic corticosteroids, including prednisone, should be used to control acute exacerbations or treat severe persistent asthma (ACOG 2008; GINA 2018; Namazy 2016).
Prednisone may be used to treat lupus nephritis in pregnant women who have active nephritis or substantial extrarenal disease activity (Hahn 2012). Prednisone is recommended for use in fetal-neonatal alloimmune thrombocytopenia and pregnancy-associated immune thrombocytopenia (ACOG 207 2019). Prednisone is the preferred immunosuppressant for the treatment of myasthenia gravis in pregnancy (Sanders 2016).
The Transplant Pregnancy Registry International (TPR) is a registry that follows pregnancies that occur in maternal transplant recipients or those fathered by male transplant recipients. The TPR encourages reporting of pregnancies following solid organ transplant by contacting them at 1-877-955-6877 or https://www.transplantpregnancyregistry.org.
Patient Education
What is this drug used for?
- It is used for many health problems like allergy signs, asthma, adrenal gland problems, blood problems, skin rashes, or swelling problems. This is not a list of all health problems that this drug may be used for. Talk with the doctor.
Frequently reported side effects of this drug
- Nausea
- Vomiting
- Trouble sleeping
- Restlessness
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Infection
- High blood sugar like confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit
- Adrenal gland problems like severe nausea, vomiting, severe dizziness, passing out, muscle weakness, severe fatigue, mood changes, lack of appetite, or weight loss
- Low potassium like muscle pain or weakness, muscle cramps, or an abnormal heartbeat
- Pancreatitis like severe abdominal pain, severe back pain, severe nausea, or vomiting
- Cushing syndrome like weight gain in upper back or abdomen; moon face; severe headache; or slow healing
- Skin changes like acne, stretch marks, slow healing, or hair growth
- DVT like swelling, warmth, numbness, change in color, or pain in the extremities
- Severe loss of strength and energy
- Fast heartbeat
- Confusion
- Sweating a lot
- Dizziness
- Passing out
- Shortness of breath
- Excessive weight gain
- Swelling of arms or legs
- Severe headache
- Fast heartbeat
- Slow heartbeat
- Abnormal heartbeat
- Chest pain
- Menstrual changes
- Joint pain
- Bone pain
- Vision changes
- Mood changes
- Behavioral changes
- Trouble with memory
- Trouble focusing
- Sensing things that seem real but are not
- Seizures
- Burning or numbness feeling
- Bruising
- Bleeding
- Severe abdominal pain
- Black, tarry, or bloody stools
- Vomiting blood
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.