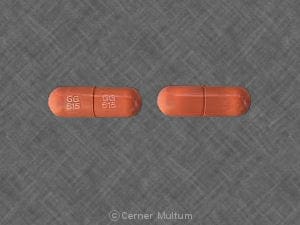
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Capsule, Oral:
Generic: 150 mg, 300 mg
Solution, Injection:
Zantac: 50 mg/2 mL (2 mL); 150 mg/6 mL (6 mL [DSC]); 1000 mg/40 mL (40 mL [DSC]) [contains phenol]
Generic: 50 mg/2 mL (2 mL); 150 mg/6 mL (6 mL); 1000 mg/40 mL (40 mL)
Syrup, Oral:
Generic: 15 mg/mL (10 mL [DSC], 473 mL, 474 mL, 480 mL [DSC]); 75 mg/5 mL (473 mL); 150 mg/10 mL (10 mL)
Tablet, Oral:
Acid Reducer: 75 mg
Acid Reducer: 75 mg, 150 mg [contains fd&c red #40 aluminum lake]
Acid Reducer: 150 mg [DSC] [sodium free, sugar free]
GoodSense Acid Reducer: 75 mg [gluten free]
raNITIdine 150 Max Strength: 150 mg [DSC] [contains fd&c blue #2 aluminum lake, fd&c red #40 aluminum lake, fd&c yellow #10 aluminum lake]
raNITIdine Acid Reducer: 75 mg
Zantac 75: 75 mg
Zantac: 150 mg [DSC], 300 mg [DSC]
Zantac 150 Maximum Strength: 150 mg
Zantac 150 Maximum Strength: 150 mg [sodium free, sugar free; contains brilliant blue fcf (fd&c blue #1); mint flavor]
Generic: 75 mg, 150 mg, 300 mg
Pharmacology
Mechanism of Action
Competitive inhibition of histamine at H2-receptors of the gastric parietal cells, which inhibits gastric acid secretion, gastric volume, and hydrogen ion concentration are reduced. Does not affect pepsin secretion, pentagastrin-stimulated intrinsic factor secretion, or serum gastrin.
Pharmacokinetics/Pharmacodynamics
Absorption
Oral: 50%; IM: Rapid
Distribution
Vd: Minimally penetrates the blood-brain barrier
Infants, Children, and Adolescents: IV, Oral: 1 to 2.3 L/kg
Adults: ~1.4 L/kg in patients with normal renal function; 1.76 L/kg in patients with CrCl 25 to 35 mL/minute
Metabolism
Hepatic (minor) to N-oxide, S-oxide, and N-desmethyl metabolites
Excretion
Urine (as unchanged drug): Oral: 30%, IV: 70%; feces (as metabolites)
Time to Peak
Serum: Oral: 2 to 3 hours; IM: ≤15 minutes
Half-Life Elimination
Neonates (receiving ECMO): IV: 6.6 hours
Infants, Children, and Adolescents: IV: 1.7 to 2.4 hours
Adults:
Oral: Normal renal function: 2.5 to 3 hours; Elderly: 3 to 4 hours
IV: Normal renal function: 2 to 2.5 hours; CrCl 25 to 35 mL/minute: 4.8 hours; Elderly: 3.1 hours
Protein Binding
~15%
Use in Specific Populations
Special Populations: Renal Function Impairment
Plasma half-life, clearance, and volume of distribution are all altered in proportion to creatinine clearance.
Special Populations: Hepatic Function Impairment
Alterations in half-life, distribution, clearance, and bioavailability are minor but clinically insignificant.
Special Populations: Elderly
Total clearance is lowered.
Special Populations: Children
In pediatric cardiac failure, clearance may be reduced. In a prospective population pharmacokinetic study of critically ill pediatric patients (n=78; age range: 15 days to 15.51 years), cardiac failure/surgery was found to reduce clearance by a factor of 0.463 (Hawwa 2013).
Use: Labeled Indications
Oral:
Gastroesophageal reflux disease: Treatment of gastroesophageal reflux disease (GERD).
Heartburn (OTC only): Relief and prevention of heartburn associated with acid indigestion and sour stomach.
Peptic ulcer disease: Treatment of active duodenal or gastric ulcers. Note: Although a labeled indication, proton pump inhibitors (PPIs) are considered the standard of care for treatment of peptic ulcer disease (PUD) rather than H2-receptor antagonists (eg, ranitidine) (Lanas 2017; Vakil 2019).
Injection:
Patients not able to take oral medication: As an alternative to the oral ranitidine dosage form for short-term (eg, GERD, peptic ulcer disease) use in patients who are unable to take oral medication.
Use: Off Label
Anaphylaxis or severe infusion reaction (adjunct to epinephrine)yes
Based on joint guidelines from the American Academy of Allergy, Asthma and Immunology (AAAAI) and the American College of Allergy, Asthma and Immunology (ACAAI) for the diagnosis and management of anaphylaxis and guidelines from the World Allergy Organization on anaphylaxis, ranitidine may be used as adjunctive treatment, but should not be used as monotherapy or as first-line therapy for anaphylaxis.
Aspiration prophylaxis in patients undergoing anesthesiabyes
Data from multiple studies of varying methodologies support the use of H2-receptor antagonists for the prevention of aspiration in patients undergoing anesthesia Manchikanti 1984, Olsson 1982, O'Reardon 2011, Stuart 1996. Studies have encompassed a wide variety of patient populations and surgical and procedural settings, including (but not limited to) elective and emergency surgeries, rapid sequence intubation, and scheduled or emergent cesarean deliveries. Clinical experience and case reports also suggest use may be especially beneficial in patients at high risk for aspiration (eg, patients with a full stomach, severe GERD, gastroparesis and/or pregnant patients undergoing anesthesia for electroconvulsive therapy [ECT]) Berkow 2019, Hagberg 2019, Kellner 2019, Nixon 2019.
Based on the American Society of Anesthesiologists (ASA) practice guidelines for obstetric anesthesia, ranitidine is an effective and recommended prophylactic agent for the prevention of aspiration during surgical procedures (eg, cesarean delivery, postpartum tubal ligation) in pregnant patients.
Chronic spontaneous urticariayes
Based on a joint guideline published by the AAAAI, ACAAI, and the Joint Council of Allergy, Asthma, & Immunology, the addition of an H2-receptor antagonist (eg, ranitidine) may be considered as adjunctive therapy to an H1 antihistamine in patients with insufficient response to a full-dose H1 antihistamine alone.
Infusion reaction, premedicationc
Clinical experience suggests the utility of H2-receptor antagonists (eg, ranitidine) as adjunctive therapy for premedication prior to the infusion of certain chemotherapy agents (eg, certain taxanes) or biologics to prevent infusion reactions Castells 2019a, Lee 2009.
Mastocytosisc
Clinical experience suggests the utility of H2-receptor antagonists (eg, ranitidine) as adjunctive therapy for cutaneous or systemic mastocytosis Akin 2018, Castells 2019b, Worobec 2000.
Scombroid (histamine) poisoningc
Clinical experience suggests the utility of H2 antagonists (eg, ranitidine) in the treatment of scombroid (histamine) poisoning Iannuzzi 2007.
Stress ulcer prophylaxis in select critically ill patientsbyes
A meta-analysis found no difference between PPIs and H2-receptor antagonists in terms of stress-related upper gastrointestinal bleeding prophylaxis, pneumonia, and mortality in intensive care units Barbateskovic 2019, Reynolds 2018.
Based on the American Society of Health-System Pharmacists (ASHP) therapeutic guidelines on stress ulcer prophylaxis, H2-receptor antagonists are recommended for stress ulcer prophylaxis in critically ill patients, although there was limited data on the use of PPIs for stress ulcer prophylaxis at the time of publication.
Based on the Surviving Sepsis Campaign international guidelines for the management of sepsis and septic shock, stress ulcer prophylaxis using an H2-receptor antagonist or a PPI is recommended in sepsis or septic shock patients who have gastrointestinal bleeding risk factors.
Contraindications
Hypersensitivity to ranitidine or any component of the formulation
OTC labeling: When used for self-medication (OTC), do not use if trouble or pain when swallowing food, vomiting with blood, or bloody or black stools, allergic to ranitidine or other acid reducers. Do not use with other acid reducers. Do not use 150 mg tablet with kidney disease without medical advice.
Documentation of allergenic cross-reactivity for histamine H2 antagonists is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Dosage and Administration
Dosing: Adult
Anaphylaxis or severe infusion reaction (adjunct to epinephrine) (off-label use):
Note: Do not use for initial or sole treatment of anaphylaxis because H2-receptor antagonists do not treat airway obstruction or shock. Although an added benefit in anaphylaxis is not well established, some experts give ranitidine after epinephrine for additional relief of hives based upon clinical experience (Campbell 2019).
IV: 50 mg as a single dose (WAO [Simons 2011]).
Aspiration prophylaxis in patients undergoing anesthesia (off-label use):
Note: May be considered in patients at high risk for aspiration (ASA 2016; Berkow 2019; Hagberg 2019; O'Reardon 2011; Rabheru 2001):
IV: 50 mg as a single dose ~40 to 90 minutes prior to induction of anesthesia; may be given with a rapid-acting nonparticulate antacid (eg, oral sodium citrate and citric acid) and/or metoclopramide (Abir 2019; ASA 2016; ASA 2017; Berkow 2019; McCammon 1986).
Oral (alternative route): 150 mg given night before surgery; repeated morning of surgery in conjunction with other appropriate agents (Abir 2019; ASA 2017).
Chronic spontaneous urticaria (alternative agent) (adjunct) (off-label use):
Note: Use as additional therapy if insufficient response to full-dose H1 antihistamine.
Oral: 150 mg twice daily given in combination with H1 antihistamine; a trial of 2 to 4 weeks is suggested to assess response (Bernstein 2014; Kahn 2019; Kulthanan 2016).
Gastroesophageal reflux disease, treatment:
Initial therapy:
Mild and intermittent symptoms (<2 episodes/week), and no evidence of erosive esophagitis:
Note: For more severe or frequent initial symptoms, with or without evidence of erosive esophagitis, a proton pump inhibitor (PPI) as initial therapy is recommended.
Oral: 75 mg twice daily as needed; if symptoms persist after 2 to 4 weeks, increase to 150 mg twice daily for 2 weeks; if symptoms improve, may continue therapy as needed (Kahrilas 2019).
Note: If symptoms persist after 2 weeks of 150 mg twice daily, discontinue and consider PPI therapy (Kahrilas 2019).
Residual acid reflux symptoms despite maximal PPI therapy (adjunct): Oral: 150 mg once daily given at bedtime in addition to PPI therapy (ACG [Katz 2013]; Iwakiri 2016); may also administer ranitidine intermittently or on-demand with scheduled PPI (Fass 2019).
OTC labeling (patient-guided therapy): Heartburn, mild intermittent symptoms: Oral: 75 to 150 mg up to twice daily when needed (maximum: 300 mg/day); may also be taken 30 to 60 minutes before meals or beverages that cause heartburn.
Infusion reaction, premedication (adjunct) (off-label use):
Typically administered 30 to 60 minutes prior to infusion of certain chemotherapy agents or biologics; usually given in conjunction with an H1 antihistamine (eg, diphenhydramine) and glucocorticoids (refer to institutional protocols) (Castells 2019a; Lee 2009).
Oral: 150 to 300 mg.
IV: 50 mg.
Mastocytosis (adjunct) (off-label use): Oral: 150 mg every 12 hours adjusted to achieve GI symptom relief. Typically used in combination with other appropriate agent(s) (eg, H1 antihistamine and/or leukotriene inhibitor) (Akin 2019; Castells 2019b; Worobec 2000).
Scombroid (histamine) poisoning (off-label use):
Note: For patients with moderate to severe symptoms, any GI symptoms, or those who have failed to respond promptly to an H1 antihistamine (eg, diphenhydramine) (Marcus 2019; Taylor 1989).
Oral: 150 mg once.
IV: 50 mg once.
Stress ulcer prophylaxis in select critically ill patients (off-label use):
Note: For ICU patients with associated risk factors for GI bleeding (including coagulopathy, mechanical ventilation for >48 hours, traumatic brain injury, history of GI ulceration or bleeding within past year, extensive burns); discontinue prophylaxis once risk factors have resolved (Rhodes 2017; Weinhouse 2019).
Oral or via nasogastric (NG) tube (alternative to enteral PPI): 150 mg twice daily (ASHP 1999; Weinhouse 2019).
IV: 50 mg every 6 to 8 hours (ASHP 1999; Weinhouse 2019).
Dosing: Geriatric
Refer to adult dosing (use caution with dose selection).
Dosing: Pediatric
Duodenal or gastric ulcer:
Treatment:
Infants, Children, and Adolescents ≤16 years:
Oral: 4 to 8 mg/kg/day divided twice daily; maximum daily dose: 300 mg/day.
IV: 2 to 4 mg/kg/day divided every 6 to 8 hours; maximum dose: 50 mg/dose.
Adolescents >16 years:
Oral:
Duodenal ulcer: 150 mg twice daily or 300 mg once daily after the evening meal or at bedtime.
Gastric ulcer: 150 mg twice daily.
IV, IM: 50 mg every 6 to 8 hours.
Maintenance:
Infants, Children, and Adolescents ≤16 years: Oral: 2 to 4 mg/kg/day once daily; maximum daily dose: 150 mg/day.
Adolescents >16 years: Oral: 150 mg once daily at bedtime.
Erosive esophagitis:
Infants, Children, and Adolescents ≤16 years: Oral: 5 to 10 mg/kg/day divided twice daily; maximum dose: 150 mg/dose.
Adolescents >16 years: Oral:
Treatment: 150 mg 4 times daily.
Maintenance: 150 mg twice daily.
GI bleed or stress ulcer; prophylaxis: Limited data available; dosing regimens variable:
Intermittent IV infusion:
Infants: 2 to 6 mg/kg/day divided every 8 hours (ASHP 1999; Crill 1999).
Children and Adolescents: 2 to 6 mg/kg/day divided every 6 hours; maximum daily dose: 300 mg/day (ASHP 1999; Crill 1999; Yildizdas 2002).
Continuous IV infusion: Infants, Children, and Adolescents: Initial: 0.15 to 0.5 mg/kg/dose for 1 dose, followed by infusion of 0.08 to 0.2 mg/kg/hour (2 to 5 mg/kg/day) (Kliegman 2007; Lugo 2001; Osteyee 1994).
Gastroesophageal reflux disease: Note: Guidelines recommend a 4- to 8-week treatment course; if improvement seen after 4 to 8 weeks, consider possible wean; if no response after 4 to 8 weeks, reevaluate diagnosis and consider referral to pediatric GI specialist (NASPGHAN/ESPGHAN [Rosen 2018]).
Infants, Children, and Adolescents: Oral: 5 to 10 mg/kg/day in 2 to 3 divided doses; maximum daily dose: 300 mg/day (AAP [Lightdale 2013]; NASPGHAN/ESPGHAN [Rosen 2018]).
Heartburn: OTC labeling: Note: Do not use for more than 14 days.
Prevention: Children ≥12 years and Adolescents: Oral: 75 to 150 mg 30 to 60 minutes before eating food or drinking beverages which cause heartburn; maximum daily dose: 2 doses/day.
Relief of symptoms: Children ≥12 years and Adolescents: Oral: 75 to 150 mg twice daily; maximum daily dose: 2 doses/day.
Pathological hypersecretory conditions (eg, Zollinger-Ellison): Note: Proton pump inhibitors are the preferred agents (Kliegman 2020).
Adolescents >16 years:
Oral: 150 mg twice daily; adjust dose or frequency as clinically indicated; doses of up to 6 g/day have been used.
Continuous IV infusion: Initial: 1 mg/kg/hour; measure gastric acid output at 4 hours, if >10 mEq/hour or if patient is symptomatic, increase dose in increments of 0.5 mg/kg/hour; doses of up to 2.5 mg/kg/hour (or 220 mg/hour) have been used.
Anaphylaxis, adjunct therapy: Note: Should not be used as monotherapy or as first-line therapy (AAAAI/ACAAI [Campbell 2014]; iCAALL [Simons 2014]; WAO [Simons 2015]).
Infants, Children, and Adolescents: IV: 1 mg/kg/dose; maximum dose: 50 mg/dose (AAAAI/ACAAI [Lieberman 2010]; Canadian Paediatric Society [Cheng 2011]).
Reconstitution
Intermittent bolus injection: Dilute in NS or other compatible IV solution to a maximum concentration of 2.5 mg/mL (20 mL).
Intermittent IV infusion: Dilute in D5W or other compatible IV solution to a maximum concentration of 0.5 mg/mL (100 mL).
IM: No dilution necessary.
Administration
Injection may be administered IM or IV:
IM: Injection is administered undiluted
IV: Must be diluted; may be administered intermittent bolus or intermittent IV infusion
Intermittent bolus: Manufacturer recommends a maximum rate of administration of 10 mg/minute (infuse over at least 5 minutes); however, in adults may also be administered at a maximum rate of 25 mg/minute (or over 2 minutes) if necessary (Coursin 1988; Goelzer 1988; Smith 1987).
Intermittent IV infusion: Administer over a maximum rate of 2.5 to 3.5 mg/minute (infuse over at least 15 to 20 minutes)
Dietary Considerations
Some products may contain phenylalanine and/or sodium. Oral dosage forms may be taken with or without food.
Storage
Capsules, tablets: Store between 20°C and 25°C (68°F and 77°F). Protect from light. Protect from moisture.
Injection: Store intact vials between 4°C and 25°C (39°F to 77°F); excursion permitted to 30°C (86°F). Protect from light; do not freeze. Avoid excessive heat (brief exposure up to 40°C does not affect the product). Undiluted solution is a clear, colorless to yellow color; slight darkening does not affect potency. Stable for 48 hours at room temperature when diluted for infusion in commonly used IV solutions (eg, NS, D5W, D10W, Ringer's lactate injection, sodium bicarbonate 5% injection).
Syrup: Store between 20°C and 25°C (68°F and 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Do not freeze. Protect from light.
Ranitidine Images
Drug Interactions
Acalabrutinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Acalabrutinib. Management: To minimize the potential for a significant interaction, separate administration of these agents by giving acalabrutinib 2 hours before ingestion of a histamine-2 receptor antagonist. Consider therapy modification
Atazanavir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Atazanavir. Management: Specific dose limitations and administration guidelines exist; consult full interaction monograph or atazanavir prescribing information. Consider therapy modification
Bosutinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Bosutinib. Management: Administer histamine H2 receptor antagonists more than 2 hours before or after bosutinib. Consider therapy modification
Cefditoren: Histamine H2 Receptor Antagonists may decrease the serum concentration of Cefditoren. Management: Concomitant use of cefditoren with H2-antagonists and antacids is not recommended. Consider alternative methods to control acid reflux (eg, diet modification) or alternative antimicrobial therapy if use of H2-antagonists can not be avoided. Consider therapy modification
Cefpodoxime: Histamine H2 Receptor Antagonists may decrease the absorption of Cefpodoxime. Separate oral doses by at least 2 hours. Monitor therapy
Cefuroxime: Histamine H2 Receptor Antagonists may decrease the absorption of Cefuroxime. Separate oral doses by at least 2 hours. Avoid combination
Cysteamine (Systemic): Histamine H2 Receptor Antagonists may diminish the therapeutic effect of Cysteamine (Systemic). Monitor therapy
Dacomitinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Dacomitinib. Management: Administer dacomitinib at least 6 hours before or 10 hours after an histamine H2-receptor antagonist (H2RA). Consider therapy modification
Dasatinib: Histamine H2 Receptor Antagonists may decrease the absorption of Dasatinib. Management: Antacids (taken 2 hours before or after dasatinib administration) can be used in place of H2-antagonists if some acid-reducing therapy is needed. Avoid combination
Delavirdine: Histamine H2 Receptor Antagonists may decrease the serum concentration of Delavirdine. Management: Chronic therapy with H2-antagonists should be avoided in patients who are being treated with delavirdine. The clinical significance of short-term H2-antagonist therapy with delavirdine is uncertain, but such therapy should be undertaken with caution. Avoid combination
Dexmethylphenidate: Histamine H2 Receptor Antagonists may increase the absorption of Dexmethylphenidate. Specifically, H2-antagonists may interfere with the normal release of drug from the extended-release capsules (Focalin XR brand), which could result in both increased absorption (early) and decreased delayed absorption. Monitor therapy
Doxofylline: RaNITIdine may increase the serum concentration of Doxofylline. Monitor therapy
Erdafitinib: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Erdafitinib: May increase the serum concentration of OCT2 Substrates. Monitor therapy
Erlotinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Erlotinib. Management: Avoid H2-antagonists in patients receiving erlotinib when possible. If concomitant treatment cannot be avoided, erlotinib should be dosed once daily, 10 hours after and at least 2 hours before H2-antagonist dosing. Consider therapy modification
Fosamprenavir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Fosamprenavir. Cimetidine may also inhibit the metabolism of the active metabolite amprenavir, making its effects on fosamprenavir/amprenavir concentrations difficult to predict. Monitor therapy
Gefitinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Gefitinib. Management: Administer gefitinib at least 6 hours before or after administration of a histamine H2-antagonist, and closely monitor clinical response to gefitinib. Consider therapy modification
Indinavir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Indinavir. Monitor therapy
Iron Preparations: Histamine H2 Receptor Antagonists may decrease the absorption of Iron Preparations. Exceptions: Ferric Carboxymaltose; Ferric Citrate; Ferric Derisomaltose; Ferric Gluconate; Ferric Hydroxide Polymaltose Complex; Ferric Pyrophosphate Citrate; Ferumoxytol; Iron Dextran Complex; Iron Sucrose. Monitor therapy
Itraconazole: Histamine H2 Receptor Antagonists may increase the serum concentration of Itraconazole. Histamine H2 Receptor Antagonists may decrease the serum concentration of Itraconazole. Management: Administer Sporanox brand itraconazole at least 2 hours before or 2 hours after administration of any histamine H2 receptor antagonists (H2RAs). Exposure to Tolsura brand itraconazole may be increased by H2RAs; consider itraconazole dose reduction. Consider therapy modification
Ketoconazole (Systemic): Histamine H2 Receptor Antagonists may decrease the serum concentration of Ketoconazole (Systemic). Management: Administer oral ketoconazole at least 2 hours prior to use of any H2-receptor antagonist. Monitor patients closely for signs of inadequate clinical response to ketoconazole. Consider therapy modification
Lasmiditan: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Avoid combination
Ledipasvir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Ledipasvir. Consider therapy modification
Lumacaftor and Ivacaftor: May decrease the serum concentration of RaNITIdine. Lumacaftor and Ivacaftor may increase the serum concentration of RaNITIdine. Monitor therapy
Mesalamine: Histamine H2 Receptor Antagonists may diminish the therapeutic effect of Mesalamine. Histamine H2-Antagonist-mediated increases in gastrointestinal pH may cause the premature release of mesalamine from specific sustained-release mesalamine products. Management: Consider avoiding concurrent administration of high-dose histamine H2-receptor antagonists with sustained-release mesalamine products. Consider therapy modification
Methylphenidate: Histamine H2 Receptor Antagonists may increase the absorption of Methylphenidate. Specifically, H2-antagonists may interfere with the normal release of drug from the extended-release capsules (Ritalin LA brand), which could result in both increased absorption (early) and decreased delayed absorption. Monitor therapy
Multivitamins/Minerals (with ADEK, Folate, Iron): Histamine H2 Receptor Antagonists may decrease the serum concentration of Multivitamins/Minerals (with ADEK, Folate, Iron). Specifically, the absorption of iron may be impaired by H2-antagonists. Monitor therapy
Nelfinavir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Nelfinavir. Concentrations of the active M8 metabolite may also be reduced. Monitor therapy
Neratinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Neratinib. Specifically, histamine H2 receptor antagonists may reduce neratinib absorption. Management: Administer neratinib at least 2 hours before or 10 hours after administration of a histamine H2 receptor antagonist to minimize the impact of this interaction. Avoid combination
Nilotinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Nilotinib. Management: The nilotinib dose should be given 10 hours after or 2 hours before the H2 receptor antagonist in order to minimize the risk of a significant interaction. Consider therapy modification
PAZOPanib: Histamine H2 Receptor Antagonists may decrease the serum concentration of PAZOPanib. Management: Avoid the use of histamine H2-antagonists in combination with pazopanib. Strategies to minimize the expected interaction between these agents (eg, dose separation) have not been investigated. Avoid combination
Pexidartinib: Histamine H2 Receptor Antagonists may decrease the serum concentration of Pexidartinib. Management: Administer pexidartinib 2 hours before or 10 hours after histamine H2 receptor antagonists. Consider therapy modification
P-glycoprotein/ABCB1 Inducers: May decrease the serum concentration of P-glycoprotein/ABCB1 Substrates. P-glycoprotein inducers may also further limit the distribution of p-glycoprotein substrates to specific cells/tissues/organs where p-glycoprotein is present in large amounts (e.g., brain, T-lymphocytes, testes, etc.). Monitor therapy
P-glycoprotein/ABCB1 Inhibitors: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. P-glycoprotein inhibitors may also enhance the distribution of p-glycoprotein substrates to specific cells/tissues/organs where p-glycoprotein is present in large amounts (e.g., brain, T-lymphocytes, testes, etc.). Monitor therapy
Posaconazole: Histamine H2 Receptor Antagonists may decrease the serum concentration of Posaconazole. Management: Avoid concurrent use of oral suspension with H2-antagonists whenever possible. Monitor patients closely for decreased antifungal effects if this combination is used. Delayed-release posaconazole tablets may be less likely to interact. Consider therapy modification
Prasugrel: RaNITIdine may decrease serum concentrations of the active metabolite(s) of Prasugrel. Monitor therapy
Procainamide: RaNITIdine may increase the serum concentration of Procainamide. Ranitidine may also increase the concentration of the active N-acetyl-procainamide (NAPA) metabolite. Monitor therapy
Ranolazine: May increase the serum concentration of P-glycoprotein/ABCB1 Substrates. Monitor therapy
Rilpivirine: Histamine H2 Receptor Antagonists may decrease the serum concentration of Rilpivirine. Management: Administer histamine H2 receptor antagonists at least 12 hours before or 4 hours after rilpivirine. Consider therapy modification
Risedronate: Histamine H2 Receptor Antagonists may increase the serum concentration of Risedronate. This applies specifically to delayed-release risedronate. Avoid combination
Saquinavir: Histamine H2 Receptor Antagonists may increase the serum concentration of Saquinavir. Monitor therapy
Secretin: Histamine H2 Receptor Antagonists may diminish the diagnostic effect of Secretin. Specifically, use of H2-Antagonists may cause a hyperresponse in gastrin secretion in response to secretin stimulation testing, falsely suggesting gastrinoma. Management: Avoid concomitant use of histamine H2-antagonists (H2RAs) and secretin. Discontinue H2RAs at least 2 days prior to secretin administration. Consider therapy modification
Sulfonylureas: RaNITIdine may increase the serum concentration of Sulfonylureas. Monitor therapy
Tafenoquine: May increase the serum concentration of OCT2 Substrates. Management: Avoid use of OCT2 substrates with tafenoquine, and if the combination cannot be avoided, monitor closely for evidence of toxicity of the OCT2 substrate and consider a reduced dose of the OCT2 substrate according to that substrate's labeling. Consider therapy modification
Triazolam: RaNITIdine may increase the serum concentration of Triazolam. Monitor therapy
Varenicline: Histamine H2 Receptor Antagonists may increase the serum concentration of Varenicline. Management: Monitor for increased varenicline adverse effects with concomitant use of cimetidine or other H2-antagonists, particularly in patients with severe renal impairment. International product labeling recommendations vary. Consult appropriate labeling. Monitor therapy
Velpatasvir: Histamine H2 Receptor Antagonists may decrease the serum concentration of Velpatasvir. Monitor therapy
Warfarin: RaNITIdine may increase the serum concentration of Warfarin. Monitor therapy
Test Interactions
False-positive urine protein using Multistix.
Adverse Reactions
Frequency not defined.
Cardiovascular: Asystole, atrioventricular block, bradycardia (with rapid IV administration), tachycardia, vasculitis, ventricular premature contractions
Central nervous system: Agitation, confusion, dizziness, depression, drowsiness, hallucination, headache, insomnia, involuntary motor activity, malaise, vertigo
Dermatologic: Alopecia, erythema multiforme, injection site pruritus (transient), skin rash
Endocrine & metabolic: Acute porphyria, increased serum prolactin
Gastrointestinal: Abdominal distress, abdominal pain, constipation, diarrhea, nausea, necrotizing enterocolitis (very low weight neonates; Guillet 2006), pancreatitis, vomiting
Hematologic & oncologic: Agranulocytosis, aplastic anemia, granulocytopenia, hemolytic anemia (immune; acquired), leukopenia, pancytopenia, thrombocytopenia
Hepatic: Cholestatic hepatitis, hepatic failure, hepatitis, jaundice
Hypersensitivity: Anaphylaxis, angioedema, hypersensitivity reaction (eg, bronchospasm, eosinophilia, fever)
Local: Burning sensation at injection site (transient), pain at injection site (transient)
Neuromuscular & skeletal: Arthralgia, myalgia
Ophthalmic: Blurred vision
Renal: Acute interstitial nephritis, increased serum creatinine
Respiratory: Pneumonia (causal relationship not established)
Warnings/Precautions
Concerns related to adverse effects:
- Confusion: Rare cases of reversible confusion have been associated with ranitidine; usually elderly or severely ill patients, or in patients with renal or hepatic impairment.
- Hepatic effects: Elevation in ALT levels has occurred with higher doses (≥100 mg) or prolonged IV therapy (≥5 days); monitor ALT levels daily for the remainder of treatment.
- Vitamin B12 deficiency: Prolonged treatment (≥2 years) may lead to vitamin B12 malabsorption and subsequent vitamin B12 deficiency. The magnitude of the deficiency is dose-related and the association is stronger in females and those younger in age (<30 years); prevalence is decreased after discontinuation of therapy (Lam 2013).
Disease-related concerns:
- Gastric malignancy: Relief of symptoms does not preclude the presence of a gastric malignancy.
- Hepatic impairment: Use with caution in patients with hepatic impairment (ranitidine undergoes hepatic metabolism).
- Porphyria: Avoid use in patients with a history of acute porphyria; may precipitate attacks.
- Renal impairment: Ranitidine is primarily excreted renally; dosage adjustment is recommended in patients with renal impairment.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Children: Use of gastric acid inhibitors, including proton pump inhibitors and H2 blockers, has been associated with an increased risk for development of acute gastroenteritis and community-acquired pneumonia in pediatric patients (Canani 2006).
Dosage form specific issues:
- Injection: Rapid administration has been associated with bradycardia (rare), usually in patients with predisposing risk factors for cardiac rhythm disorders. Do not exceed the recommended IV administration rate(s).
- Syrup: May contain up to 7.5% alcohol.
Other warnings/precautions:
- OTC labeling: When used for self-medication (OTC), notify health care provider before use if any of the following are present: Frequent chest pain; frequent wheezing particularly with heartburn; nausea/vomiting; unexplained weight loss; stomach pain; heartburn longer than 3 months; heartburn with light-headedness, sweating, or dizziness; chest pain or shoulder pain with shortness of breath; sweating or pain that spreads to arms, neck, or shoulders; light-headedness. Stop use and notify health care provider if heartburn continues, worsens, or lasts longer than 14 days.
Monitoring Parameters
AST, ALT, serum creatinine; occult blood with GI bleeding, signs/symptoms of peptic ulcer disease; when used to prevent stress-related GI bleeding, measure the intragastric pH and try to maintain pH >4; when used for Zollinger-Ellison syndrome, monitor gastric acid secretion (goal: <10 mEq/hour); signs of confusion
Pregnancy
Pregnancy Considerations
Ranitidine crosses the placenta (Armentano 1989). Histamine H2 antagonists have been evaluated for the treatment of gastroesophageal reflux disease (GERD) as well as gastric and duodenal ulcers during pregnancy. If needed, ranitidine is the agent of choice (Cappell 2003; Richter 2003). Histamine H2 antagonists may be used for aspiration prophylaxis prior to cesarean delivery (ASA 2016).
Patient Education
What is this drug used for?
- It is used to treat gastroesophageal reflux disease (GERD; acid reflux).
- It is used to treat or prevent GI (gastrointestinal) ulcers.
- It is used to treat heartburn and sour stomach.
- It is used to treat syndromes caused by lots of stomach acid.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Headache
- Nausea
- Vomiting
- Constipation
- Diarrhea
- Abdominal pain
- Injection site irritation
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.