Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral, as fumarate:
Mayzent: 0.25 mg, 2 mg [contains soybean lecithin]
Tablet Therapy Pack, Oral, as fumarate:
Mayzent Starter Pack: 0.25 mg (12 ea) [contains soybean lecithin]
Pharmacology
Mechanism of Action
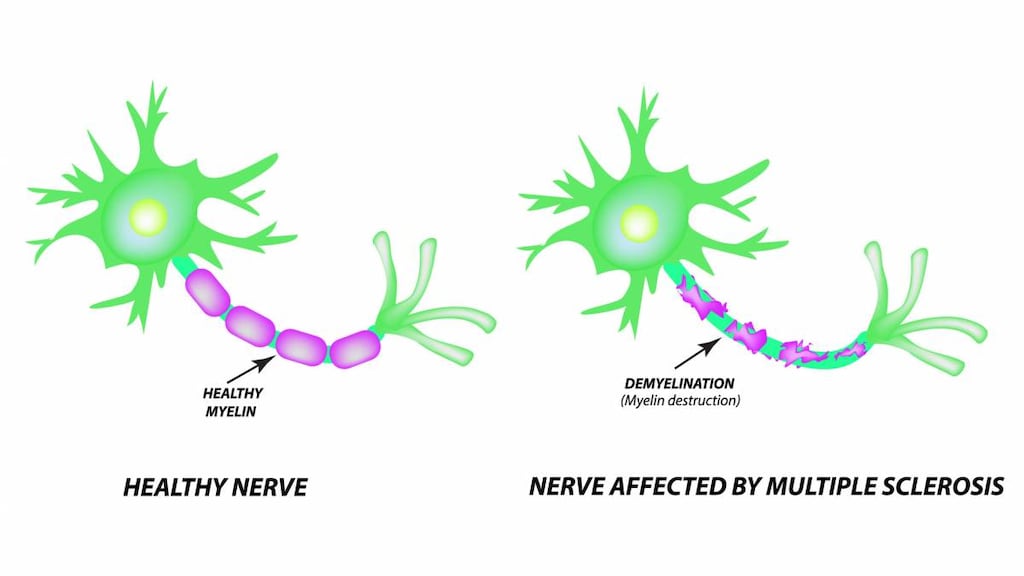
Siponimod, a sphingosine-1-phosphate (S1P) receptor modulator, binds to sphingosine 1-phosphate receptors 1 and 5. Siponimod blocks the lymphocytes' ability to emerge from lymph nodes; therefore, the amount of lymphocytes available to the CNS is decreased, which reduces central inflammation (Behrangi 2019).
Pharmacokinetics/Pharmacodynamics
Absorption
Extensive.
Distribution
Vd: 124 L.
Metabolism
Extensively metabolized, mainly via CYP2C9 (79.3%), followed by CYP3A4 (18.5%) to inactive metabolites, M3 and M17.
Excretion
Biliary/fecal (as inactive metabolites).
Time to Peak
~4 hours (range 3 to 8 hours).
Half-Life Elimination
~30 hours.
Protein Binding
>99.9%.
Use: Labeled Indications
Multiple sclerosis: Treatment of relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
Contraindications
CYP2C9*3/*3 genotype; recent (in the past 6 months) MI, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization, or Class III or IV heart failure; Mobitz type II second-degree, third-degree AV block, or sick sinus syndrome, unless patient has a functioning pacemaker.
Dosage and Administration
Dosing: Adult
Note: Before initiating siponimod, determine CYP2C9 genotype and dose accordingly. First-dose 6-hour monitoring is recommended for patients with certain preexisting cardiac conditions, including sinus bradycardia (HR <55 bpm), first- or second-degree (Mobitz type 1) AV block, or a history of MI or heart failure. For these patients, administer the first dose and doses following therapy interruption (≥4 days) in a setting in which resources to appropriately manage symptomatic bradycardia and other conduction abnormalities are available.
Multiple sclerosis: Oral:
CYP2C9 Genotype *1/*1, *1/*2, or *2/*2:
Initial: 0.25 mg once daily on Days 1 and 2, then 0.5 mg once daily on Day 3, then 0.75 mg once daily on Day 4, then 1.25 mg once daily on Day 5.
Maintenance: 2 mg once daily, beginning on Day 6.
CYP2C9 Genotype *1/*3 or *2/*3:
Initial: 0.25 mg once daily on Days 1 and 2, then 0.5 mg once daily on Day 3, then 0.75 mg once daily on Day 4.
Maintenance: 1 mg once daily, beginning on Day 5.
Missed dose: If a dose is missed for more than 24 hours during the initial titration regimen, reinitiate with Day 1 of the titration regimen. If treatment with siponimod is interrupted for 4 or more consecutive daily doses after completion of initial titration, reinitiate treatment with Day 1 of the titration regimen, including first-dose monitoring when appropriate.
Dosing: Geriatric
Refer to adult dosing.
Administration
Oral: Administer with or without food.
Storage
Store unopened containers in a refrigerator between 2°C to 8°C (36°F to 46°F).
Store opened containers as follows:
Starter Pack/Blister Card: 20°C to 25°C (68°F to 77°F) for ≤1 week after opening the blister. Store in original container.
Bottles: 20°C to 25°C (68°F to 77°F) for ≤1 month after opening the bottles.
Drug Interactions
Alpelisib: May decrease the serum concentration of CYP2C9 Substrates (High risk with Inducers). Monitor therapy
Baricitinib: Immunosuppressants may enhance the immunosuppressive effect of Baricitinib. Management: Use of baricitinib in combination with potent immunosuppressants such as azathioprine or cyclosporine is not recommended. Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted. Consider therapy modification
BCG (Intravesical): Immunosuppressants may diminish the therapeutic effect of BCG (Intravesical). Avoid combination
Bradycardia-Causing Agents: May enhance the bradycardic effect of Siponimod. Management: Avoid coadministration of siponimod with drugs that may cause bradycardia. Consider therapy modification
Calcimimetic Agents: May increase the serum concentration of Siponimod. Management: Coadministration of siponimod with drugs which are both moderate inhibitors of CYP2C9 and moderate or strong inhibitors of CYP3A4 is not recommended. Consider therapy modification
Ceritinib: Bradycardia-Causing Agents may enhance the bradycardic effect of Ceritinib. Management: If this combination cannot be avoided, monitor patients for evidence of symptomatic bradycardia, and closely monitor blood pressure and heart rate during therapy. Exceptions are discussed in separate monographs. Consider therapy modification
Cladribine: May enhance the immunosuppressive effect of Immunosuppressants. Avoid combination
Coccidioides immitis Skin Test: Immunosuppressants may diminish the diagnostic effect of Coccidioides immitis Skin Test. Monitor therapy
CYP2C9 Inhibitors (Moderate): May increase the serum concentration of Siponimod. Monitor therapy
Dabrafenib: May decrease the serum concentration of CYP2C9 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP2C9 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Denosumab: May enhance the adverse/toxic effect of Immunosuppressants. Specifically, the risk for serious infections may be increased. Monitor therapy
Echinacea: May diminish the therapeutic effect of Immunosuppressants. Consider therapy modification
Enzalutamide: May decrease the serum concentration of Siponimod. Management: Coadministration of siponimod with enzalutamide, a moderate inducers of CYP2C9 and a strong inducer of CYP3A4, is not recommended. Avoid combination
Fexinidazole [INT]: Bradycardia-Causing Agents may enhance the arrhythmogenic effect of Fexinidazole [INT]. Avoid combination
Fingolimod: Immunosuppressants may enhance the immunosuppressive effect of Fingolimod. Management: Avoid the concomitant use of fingolimod and other immunosuppressants when possible. If combined, monitor patients closely for additive immunosuppressant effects (eg, infections). Consider therapy modification
Fluconazole: May increase the serum concentration of Siponimod. Management: Coadministration of siponimod with fluconazole, a moderate inhibitor of CYP2C9 and a moderate inhibitor of CYP3A4 is not recommended. Avoid combination
Haloperidol: QT-prolonging Agents (Indeterminate Risk - Caution) may enhance the QTc-prolonging effect of Haloperidol. Monitor therapy
Immunosuppressants: May enhance the immunosuppressive effect of Siponimod. Monitor therapy
Lacosamide: Bradycardia-Causing Agents may enhance the AV-blocking effect of Lacosamide. Monitor therapy
Leflunomide: Immunosuppressants may enhance the adverse/toxic effect of Leflunomide. Specifically, the risk for hematologic toxicity such as pancytopenia, agranulocytosis, and/or thrombocytopenia may be increased. Management: Consider not using a leflunomide loading dose in patients receiving other immunosuppressants. Patients receiving both leflunomide and another immunosuppressant should be monitored for bone marrow suppression at least monthly. Consider therapy modification
Lumacaftor and Ivacaftor: May decrease the serum concentration of CYP2C9 Substrates (High Risk with Inhibitors or Inducers). Lumacaftor and Ivacaftor may increase the serum concentration of CYP2C9 Substrates (High Risk with Inhibitors or Inducers). Monitor therapy
Midodrine: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
MiFEPRIStone: May increase the serum concentration of Siponimod. Management: Coadministration of siponimod with miferpristone, a moderate inhibitor of CYP2C9 and a strong inhibitor of CYP3A4 is not recommended. Avoid combination
Natalizumab: Immunosuppressants may enhance the adverse/toxic effect of Natalizumab. Specifically, the risk of concurrent infection may be increased. Avoid combination
Nivolumab: Immunosuppressants may diminish the therapeutic effect of Nivolumab. Consider therapy modification
Ocrelizumab: May enhance the immunosuppressive effect of Immunosuppressants. Monitor therapy
Pidotimod: Immunosuppressants may diminish the therapeutic effect of Pidotimod. Monitor therapy
Pimecrolimus: May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
QT-prolonging Agents (Highest Risk): QT-prolonging Agents (Indeterminate Risk - Caution) may enhance the QTc-prolonging effect of QT-prolonging Agents (Highest Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
RifAMPin: May decrease the serum concentration of Siponimod. Management: Coadministration of siponimod with rifampin, a moderate inducer of CYP2C9 and a strong inducer of CYP3A4, is not recommended. Avoid combination
Rifapentine: May decrease the serum concentration of CYP2C9 Substrates (High risk with Inducers). Monitor therapy
Roflumilast: May enhance the immunosuppressive effect of Immunosuppressants. Consider therapy modification
Ruxolitinib: May enhance the bradycardic effect of Bradycardia-Causing Agents. Management: Ruxolitinib Canadian product labeling recommends avoiding use with bradycardia-causing agents to the extent possible. Monitor therapy
Sipuleucel-T: Immunosuppressants may diminish the therapeutic effect of Sipuleucel-T. Management: Evaluate patients to see if it is medically appropriate to reduce or discontinue therapy with immunosuppressants prior to initiating sipuleucel-T therapy. Consider therapy modification
Tacrolimus (Topical): May enhance the adverse/toxic effect of Immunosuppressants. Avoid combination
Terlipressin: May enhance the bradycardic effect of Bradycardia-Causing Agents. Monitor therapy
Tertomotide: Immunosuppressants may diminish the therapeutic effect of Tertomotide. Monitor therapy
Tofacitinib: Immunosuppressants may enhance the immunosuppressive effect of Tofacitinib. Management: Concurrent use with antirheumatic doses of methotrexate or nonbiologic disease modifying antirheumatic drugs (DMARDs) is permitted, and this warning seems particularly focused on more potent immunosuppressants. Consider therapy modification
Trastuzumab: May enhance the neutropenic effect of Immunosuppressants. Monitor therapy
Upadacitinib: Immunosuppressants may enhance the immunosuppressive effect of Upadacitinib. Avoid combination
Vaccines (Inactivated): Siponimod may diminish the therapeutic effect of Vaccines (Inactivated). Management: Avoid administration of vaccines (inactivated) during treatment with siponimod and for 1 month after discontinuation due to potential decreased vaccine efficacy. Consider therapy modification
Vaccines (Live): Immunosuppressants may enhance the adverse/toxic effect of Vaccines (Live). Immunosuppressants may diminish the therapeutic effect of Vaccines (Live). Management: Avoid use of live organism vaccines with immunosuppressants; live-attenuated vaccines should not be given for at least 3 months after immunosuppressants. Exceptions: Smallpox and Monkeypox Vaccine (Live). Avoid combination
Vaccines (Live): Siponimod may enhance the adverse/toxic effect of Vaccines (Live). Siponimod may diminish the thrombocytopenic effect of Vaccines (Live). Management: Avoid administration of vaccine (live) during treatment with siponimod and for 1 month after discontinuation due to potential decreased vaccine efficacy and increased infection risk. Consider therapy modification
Adverse Reactions
>10%:
Cardiovascular: Hypertension (13%)
Central nervous system: Headache (15%), falling (11%)
Hepatic: Increased serum transaminases (≤11%)
1% to 10%:
Cardiovascular: Peripheral edema (8%), bradycardia (4% to 6%), first degree atrioventricular block (5%), second degree atrioventricular block (<2%)
Central nervous system: Dizziness (7%), seizure (2% to <5%)
Gastrointestinal: Nausea (7%), diarrhea (6%)
Hematologic & oncologic: Lymphocytopenia (<5%)
Hepatic: Increased serum bilirubin (≤10%), increased serum alanine aminotransferase (6%), increased serum aspartate aminotransferase (1%)
Infection: Herpes virus infection (5%), herpes zoster infection (<5%)
Neuromuscular & skeletal: Limb pain (6%), asthenia (<5%), tremor (<5%)
Ophthalmic: Macular edema (2% to <5%)
Respiratory: Reduced forced expiratory volume (<5%)
Frequency not defined:
Dermatologic: Facial swelling
Infection: Infection
<1%, postmarketing, and/or case reports: Malignant melanoma, meningitis (cryptococcal), testicular neoplasm (seminoma), varicella zoster infection (meningitis)
Warnings/Precautions
Concerns related to adverse effects:
- Atrioventricular conduction delays: Initiation has been associated with transient and asymptomatic AV conduction delays, including first-degree atrioventricular (AV) block (most cases) and second-degree AV block, usually Mobitz type I. The conduction abnormalities typically occur concomitantly with bradycardia, resolve within 24 hours of treatment initiation, and do not require discontinuation of siponimod or treatment with atropine. No second-degree AV blocks of Mobitz type II or higher degree were observed, and most conduction abnormalities occurred at doses >2 mg or in situations when the siponimod dose was not titrated.
- Bradycardia: Initiation results in transient decreases in heart rate; gradual titration will minimize this effect. Following the first titration dose, heart rate may decrease as soon as 1 hour postdose, with the maximal decrease usually occurring ~3 to 4 hours postdose. Although postdose bradycardia will likely continue to occur during the dose titration period, it will not be as pronounced as day 1. Heart rate begins to increase after day 6 of therapy and typically returns to baseline after 10 days of therapy. Most patients are asymptomatic; however, dizziness and fatigue may occur; symptoms usually resolve within 24 hours.
- Hepatic effects: Elevated liver enzymes may occur; most elevations occurred within 6 months of treatment initiation. Obtain baseline liver enzymes and bilirubin in all patients prior to therapy initiation (within 6 months); monitor liver enzymes in patients who develop symptoms of hepatic dysfunction (eg, nausea, vomiting, abdominal pain, fatigue, anorexia, jaundice, dark urine). Discontinue treatment with confirmation of liver injury; transaminases tend to return to normal within 1 month of discontinuation.
- Hypertension: Increased blood pressure may occur ~1 month after initiation of therapy; monitor blood pressure throughout treatment.
- Infections: Dose-dependent reduction of peripheral lymphocyte counts may increase risk of infections; life-threatening and rare fatal infections have occurred. Review CBC before treatment initiation. Delay treatment initiation in patients with severe active infections. Peripheral lymphocyte counts may also be lowered for 3 to 4 weeks after treatment discontinuation; therefore, continue to monitor for signs of infection during this period. Consider suspension of treatment if a serious infection develops. Rare cases of cryptococcal meningitis have occurred with siponimod; disseminated cryptococcal infections have been reported with another sphingosine 1-phosphate receptor modulator. In patients with signs and symptoms of cryptococcal infection, treatment with siponimod should be interrupted until cryptococcal infection has been ruled out. Herpes viral infections, including reactivation of varicella zoster virus (VZV) infection, have also occurred.
- Macular edema: Macular edema has been reported, typically within the first 4 months of treatment. Patients may present with blurred vision, decreased visual acuity, or without symptoms. Patients with a history of diabetes mellitus or uveitis are at increased risk; use with caution. Perform ophthalmologic exams (including the fundus and macula) at baseline and if vision changes; more frequent examination is warranted in patients with diabetes or a history of uveitis.
- Malignancy: Cases of lymphoma and skin cancer have been reported with another sphingosine 1-phosphate receptor modulator (Manouchehri 2018; Robinson 2016).
- Neurotoxicity: Posterior reversible encephalopathy syndrome (PRES) has been observed with another sphingosine 1-phosphate receptor modulator. Monitor for signs/symptoms of PRES (eg, behavioral changes, cognitive deficits, cortical visual disturbances, any other neurological cortical symptom/sign, or symptoms/signs suggestive of increased intracranial pressure); symptoms are usually reversible, but may evolve into ischemic stroke or cerebral hemorrhage. Delayed diagnosis and treatment may result in permanent neurological sequelae. Discontinue use if PRES is suspected.
- Progressive multifocal leukoencephalopathy: Cases of progressive multifocal leukoencephalopathy (PML) due to the John Cunningham (JC) virus have been reported with another sphingosine 1-phosphate receptor modulator and have been associated with specific risk factors, including immune compromise and immunosuppressant polypharmacy. Symptoms progress over days to weeks and may include progressive weakness on one side of the body or clumsiness of limbs, vision disturbances, and mental status changes. At the first sign or symptom suggestive of PML, perform a diagnostic evaluation and withhold therapy. MRI findings may be apparent before patients are symptomatic. Monitoring with brain MRI for signs that may be consistent with PML may be beneficial and allow for an early diagnosis of PML.
- QT prolongation: May cause QT prolongation; patients with a prolonged QT interval (>500 msec) at baseline or during the first 6 hours of treatment initiation, at an increased risk of QT prolongation, or on concomitant QT-prolonging drugs may require continuous overnight ECG monitoring in a medical facility after the initial dose.
- Respiratory effects: Reductions of forced expiratory volume in the first second of expiration (FEV1) are dose dependent and may occur within the first 3 months of therapy. It is unknown whether these changes are reversible with drug discontinuation. If clinically necessary, spirometric evaluation of respiratory function should be performed during therapy.
Disease-related concerns:
- Cardiovascular disease: For patients with sinus bradycardia (HR <55 bpm), first- or second-degree (Mobitz type 1) AV block, or a history of MI or heart failure (HF), initiation must occur in a setting with resources and personnel capable of appropriately managing symptomatic bradycardia. Patients may also require overnight monitoring during treatment initiation if they have prolonged QTc interval at baseline or at 6-hour postdose, ECG, additional risks for QT prolongation, concurrent therapy with QT prolonging agents with a known risk of torsades de pointes or drugs that slow heart rate or AV conduction, or some preexisting heart and cerebrovascular conditions. Consult with a cardiologist before initiating siponimod in patients with QTc >500 msec, arrhythmias requiring treatment with Class Ia or Class III antiarrhythmic drugs, ischemic heart disease, HF, history of cardiac arrest or MI, cerebrovascular disease, uncontrolled hypertension, history of second degree Mobitz type II or higher AV block, sick-sinus syndrome, or sino-atrial heart block.
- Hepatic impairment: Use with caution and closely monitor patients with significant hepatic disease; may be at increased risk of increased liver enzymes.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Other warnings/precautions:
- Discontinuation of therapy: Cases of rebound syndrome (clinical and radiological signs of severe exacerbation beyond what was expected) have been reported with discontinuation of another sphingosine 1-phosphate receptor modulator. Monitor for development of severe increase in disability following discontinuation and begin appropriate treatment as needed. Due to residual pharmacodynamic effects following treatment discontinuation (eg, decreased peripheral lymphocyte counts), use caution for 3 to 4 weeks after the last dose of therapy.
- Immunizations: Testing for antibodies to varicella zoster virus (VZV) is recommended prior to initiation of treatment if history of chickenpox or VZV vaccination status is unknown. Complete full course of immunization for antibody-negative patients with varicella vaccine at least 4 weeks prior to initiating treatment. Avoid immunization with live attenuated vaccines during and for 4 weeks after treatment. Vaccines may not be as effective if administered during therapy; discontinuation of treatment 1 week before and 4 weeks following a planned vaccination is recommended.
Monitoring Parameters
All patients:
CBC including lymphocyte counts (baseline [within 6 months or after discontinuation of previous therapy])
Hepatic monitoring: Baseline bilirubin and transaminase levels in all patients prior to therapy initiation (within 6 months); monitor transaminases in patients who develop symptoms of hepatic dysfunction.
ECG (baseline); ophthalmologic exam of fundus, including macula (at baseline and if vision changes, more frequent in patients with diabetes or a history of uveitis), respiratory function (FEV1) if clinically indicated, VZV antibodies (prior to starting treatment in patients with no health care professional-confirmed history of chickenpox or without documented previous full series VZV vaccination), blood pressure, signs and symptoms of infection (during treatment and at least 3 to 4 weeks after discontinuation), signs/symptoms of progressive multifocal leukoencephalopathy, and/or posterior reversible encephalopathy syndrome; monitor for suspicious skin lesions; severe increase in disability following discontinuation of therapy.
Additional required monitoring for patients with sinus bradycardia (HR <55 bpm), first- or second-degree (Mobitz type 1) AV block, or a history of MI or heart failure:
First-dose 6-hour monitoring: Monitor patient for 6 hours following the first dose for signs and symptoms of bradycardia; assess heart rate and blood pressure measurements every 1 hour. Repeat ECG after initial 6-hour dose observation period. After the initial 6-hour monitoring, continue to monitor (until resolution) if 6-hour postdose heart rate is <45 bpm, 6-hour postdose heart rate is lowest postbaseline measurement, or 6-hour postdose ECG shows new-onset second-degree or higher AV block. Start continuous ECG monitoring if postdose symptomatic bradycardia, bradyarrhythmia, or conduction-related symptoms occur or if 6-hour postdose ECG shows new-onset second degree or higher AV block or QTc ≥500 msec. Monitor until symptom resolution if no pharmacologic treatment is necessary. Monitor overnight with continuous ECG in a medical facility and repeat observation period for second dose if pharmacologic intervention is necessary.
Patients may also require overnight monitoring during treatment initiation if they have prolonged QTc interval at baseline or at 6-hour postdose ECG, additional risks for QT prolongation, concurrent therapy with QT prolonging agents with a known risk of torsades de pointes, concurrent therapy with drugs that slow heart rate or AV conduction, or some preexisting heart and cerebrovascular conditions.
Initial monitoring procedures (ECG, heart rate, blood pressure) must be repeated for:
- treatment interruption of 24 hours during the initial titration regimen, or
- treatment interruption of ≥4 consecutive days during the maintenance period
Pregnancy
Pregnancy Considerations
Based on the mechanism of action and data from animal reproduction studies, in utero exposure to siponimod may cause fetal harm. Disease modifying therapies are generally not initiated during pregnancy (AAN [Rae-Grant 2018]).
Females of reproductive potential should use effective contraception during therapy and for 10 days after the last siponimod dose.
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience nausea, diarrhea, or painful extremities. Have patient report immediately to prescriber signs of infection, signs of meningitis (headache with fever, stiff neck, nausea, confusion, or sensitivity to light), signs of liver problems (dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin), severe headache, dizziness, passing out, vision changes, shortness of breath, difficulty breathing, swelling in the arms or legs, signs of posterior reversible encephalopathy syndrome (confusion, not alert, vision changes, seizures, or severe headache), or signs of progressive multifocal leukoencephalopathy (confusion, depression, trouble with memory, behavioral changes, change in strength on one side is greater than the other, difficulty speaking, change in balance, or vision changes) (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.