Boxed Warning
Prolonged tocolysis:
Terbutaline has not been approved and should not be used for prolonged tocolysis (beyond 48 to 72 hours). In particular, terbutaline should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline to pregnant women. In mothers, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema, and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Solution, Injection, as sulfate:
Generic: 1 mg/mL (1 mL)
Solution, Injection, as sulfate [preservative free]:
Generic: 1 mg/mL (1 mL)
Tablet, Oral, as sulfate:
Generic: 2.5 mg, 5 mg
Pharmacology
Mechanism of Action
Relaxes bronchial and uterine smooth muscle by action on beta2-receptors with less effect on heart rate
Pharmacokinetics/Pharmacodynamics
Absorption
33% to 50%
Metabolism
Hepatic to inactive sulfate conjugates
Excretion
Urine (60% as unchanged drug); feces
Onset of Action
Oral: 30 to 45 minutes; SubQ: 6 to 15 minutes; Inhalation: 5 minutes (maximum effect: 15 to 60 minutes)
Time to Peak
Serum: SubQ: 0.5 hours
Duration of Action
Oral: 4 to 8 hours; Oral inhalation: 3 to 6 hours; SubQ: 1.5 to 4 hours
Half-Life Elimination
5.7 hours (range: 2.9 to 14 hours)
Protein Binding
25%
Use: Labeled Indications
Asthma/Bronchospasm: Prevention and reversal of bronchospasm in patients ≥12 years of age with asthma and reversible bronchospasm associated with bronchitis and emphysema
Use: Off Label
Extravasation management, sympathomimetic vasoconstrictorsc
Data from a limited number of patients suggests that terbutaline may be beneficial for the management of extravasation of sympathomimetic vasoconstrictors Stier 1999. Additional data may be necessary to further define the role of terbutaline in this condition.
Premature labor (acute; short-term [≤72 hours] tocolysis)yes
The American College of Obstetricians and Gynecologists (ACOG) recommends that terbutaline may be used as a tocolytic agent (short-term [≤72 hours]) in prevention or management of preterm labor ACOG 2016.
Contraindications
Hypersensitivity to terbutaline, sympathomimetic amines, or any component of the formulation
Injection: Additional contraindications: Prolonged (>48 to 72 hours) tocolysis, especially for maintenance in the outpatient setting
Oral: Additional contraindications: Acute or maintenance tocolysis
Bricanyl Turbuhaler [Canadian product]: History of tachyarrhythmias; as tocolytic in patients at risk of premature labor or threatened abortion.
Dosage and Administration
Dosing: Adult
Asthma/bronchospasm:
Oral: 5 mg 3 times daily (approximately every 6 hours); reduce dose to 2.5 mg 3 times daily if side effects occur; maximum: 15 mg/24 hours
SubQ: 0.25 mg/dose; may repeat every 20 minutes for 3 doses (maximum: 0.75 mg/1-hour period) (NAEPP 2007)
Manufacturer's labeling: Dosing in the prescribing information may not reflect current clinical practice. 0.25 mg/dose; may repeat in 15 to 30 minutes (maximum: 0.5 mg/4-hour period)
Inhalation: Bricanyl Turbuhaler [Canadian product]: One inhalation (0.5 mg) as needed; if not effective after 5 minutes may repeat dose. If second dose is not effective, consult healthcare provider immediately. Additional doses may be administered however >6 inhalations in a 24 hour period should not be needed. Note: If adequate relief is not obtained with previously effective dose, or if effects of inhalation last <3 hours, patient should be reassessed promptly; may indicate worsening asthma.
Extravasation management, sympathomimetic vasoconstrictors (off-label use; based on limited case reports): SubQ:
Large extravasations: Infiltrate extravasation area using a solution of 1 mg diluted in 10 mL of 0.9% sodium chloride; volume of terbutaline solution administered varied from 3 to 10 mL (Stier 1999).
Small/distal extravasations: Infiltrate extravasation area using a solution of 1 mg diluted in 1 mL of 0.9% sodium chloride; volume of terbutaline solution administered varied from 0.5 to 1 mL (Stier 1999).
Premature labor (acute; short-term [≤72 hours] tocolysis) (off-label use):
IV: 2.5 to 5 mcg/minute; increased gradually every 20 to 30 minutes by 2.5 to 5 mcg/minute up to a maximum of 25 mcg/minute; decrease to the lowest effective dose once contractions are controlled (Mackeen 2014; Travis 1993).
SubQ: 0.25 mg every 20 minutes to 3 hours; hold for pulse >120 beats per minute. Terbutaline has not been approved for and should not be used for prolonged tocolysis (beyond 48 to 72 hours) (ACOG 171 2016; Hearne 2000).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Asthma, acute exacerbation:
Continuous infusion: Limited data available, optimal dose not defined, efficacy results variable: Children and Adolescents: Initial: IV bolus dose: 4 to 10 mcg/kg followed by continuous infusion of 0.2 to 0.4 mcg/kg/minute, titrate by 0.1 to 0.2 mcg/kg/minute increments as frequently as every 30 minutes based on patient response or toxicity. Usual maximum dose is 5 mcg/kg/minute; however, doses as high as 10 mcg/kg/minute have been described; monitor closely for adverse reactions (Bogie 2007; Carroll 2006; Doymaz 2018; Kambalapalli 2005; Singhi 2014; Stephanopoulos 1998).
Oral:
Children ≥12 and Adolescents <15 years: 2.5 mg three times daily; maximum daily dose: 7.5 mg/24 hours
Adolescents ≥15 years:5 mg three times daily (approximately every 6 hours); reduce dose to 2.5 mg three times daily if side effects occur; maximum daily dose: 15 mg/24 hours
SubQ:
Children: Limited data available: SubQ: 0.01 mg/kg/dose every 20 minutes for 3 doses; may repeat every 2 to 6 hours as needed (NAEPP 2007)
Adolescents:SubQ: 0.25 mg/dose; may repeat every 20 minutes for up to 3 doses (NAEPP 2007)
Oral inhalation: Canadian labeling: Bricanyl Turbuhaler [Canadian product]: Children ≥6 years and Adolescents: 1 inhalation (0.5 mg) as needed; if not effective after 5 minutes may repeat dose. If second dose is not effective, consult health care provider immediately. Maximum daily dose: 6 inhalations/24 hours.
Reconstitution
For extravasation management (off-label use): Using vial for injection, dilute 1 mg in 10 mL (large extravasation site) or 1 mg in 1 mL (small/distal extravasation site) of 0.9% sodium chloride (Stier 1999).
Extemporaneously Prepared
A 1 mg/mL oral suspension may be made with tablets. Crush twenty-four 5 mg tablets in a mortar and reduce to a fine powder. Add 5 mL purified water USP and mix to a uniform paste; mix while adding simple syrup, NF in incremental proportions to almost 120 mL; transfer to a calibrated bottle, rinse mortar with vehicle, and add quantity of simple syrup, NF sufficient to make 120 mL. Label "shake well" and "refrigerate". Stable for 30 days.
Nahata MC, Pai VB, and Hipple TF, Pediatric Drug Formulations, 5th ed, Cincinnati, OH: Harvey Whitney Books Co, 2004.
Administration
IV: Use infusion pump.
Oral: Administer around-the-clock to promote less variation in peak and trough serum levels
Inhalation: Bricanyl Turbuhaler [Canadian product]: After removing lid, patient should hold inhaler upright and turn blue grip as far as it will go in one direction then turn it back to original position. Clicking sound indicates that inhaler is ready for use. Patient should exhale fully but not into the inhaler and then place mouthpiece gently between teeth, close lips around inhaler and inhale deeply. Inhaler should be removed from mouth prior to exhaling. Instruct patients to rinse mouth with water after each inhalation as some medication may stick to the inside of the mouth and throat. If inhaler is dropped or shaken, or if patient exhales into the inhaler after a dose is loaded, the dose will be lost and a new dose should be loaded and inhaled. Outside of mouthpiece should be cleaned once weekly with a dry tissue. Instruct patient to keep inhaler dry. First appearance of red mark in dose indicator (window underneath mouthpiece) indicates that 20 doses remain. When red mark reaches bottom of dose indicator no doses remain and Turbuhaler should be discarded.
SubQ: Extravasation management, sympathomimetic vasopressors (off-label use): Stop vesicant infusion immediately and disconnect IV line (leave needle/cannula in place); gently aspirate extravasated solution from the IV line (do NOT flush the line); remove needle/cannula; elevate extremity. Infiltrate extravasation area with terbutaline solution 1 mg diluted with 10 mL (large extravasation site) or 1 mg diluted in 1 mL (small/distal extravasation site) of 0.9% sodium chloride into extravasation site (Stier 1999).
Storage
Store injection at room temperature; do not freeze. Protect from heat and light. Use only clear solutions. Store powder for inhalation (Bricanyl® Turbuhaler [Canadian availability]) at room temperature between 15°C and 30°C (58°F and 86°F).
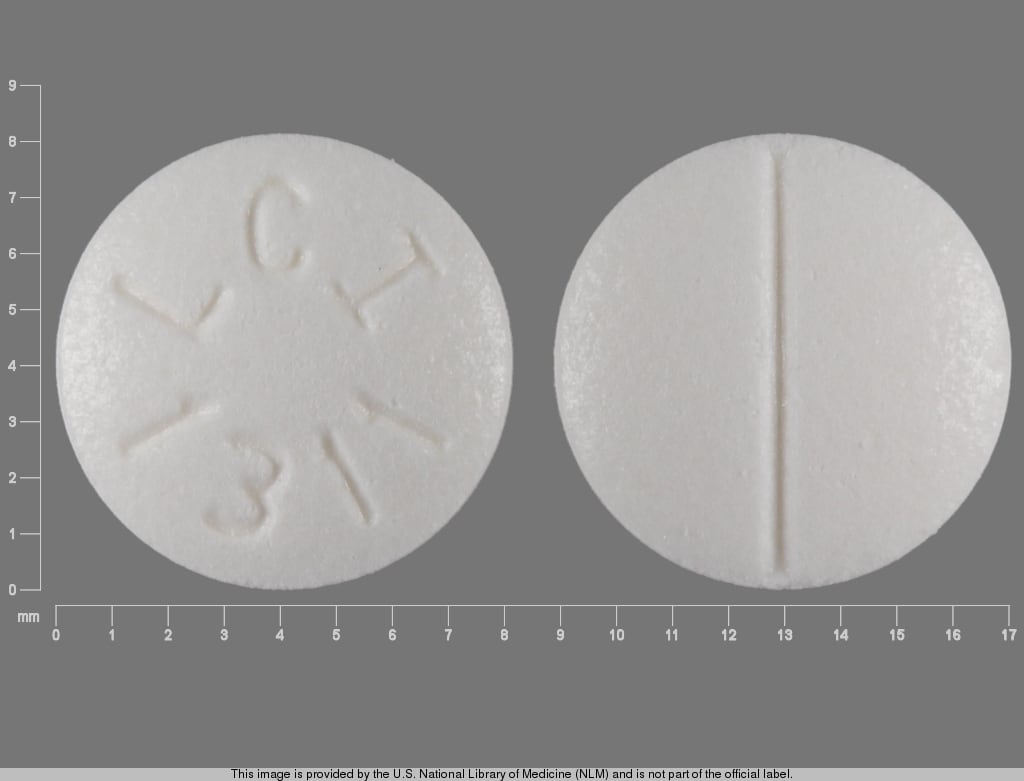
Terbutaline Images
Drug Interactions
AtoMOXetine: May enhance the tachycardic effect of Beta2-Agonists. Monitor therapy
AtoMOXetine: May enhance the hypertensive effect of Sympathomimetics. AtoMOXetine may enhance the tachycardic effect of Sympathomimetics. Monitor therapy
Atosiban: Beta2-Agonists may enhance the adverse/toxic effect of Atosiban. Specifically, there may be an increased risk for pulmonary edema and/or dyspnea. Monitor therapy
Beta-Blockers (Beta1 Selective): May diminish the bronchodilatory effect of Beta2-Agonists. Of particular concern with nonselective beta-blockers or higher doses of the beta1 selective beta-blockers. Monitor therapy
Beta-Blockers (Nonselective): May diminish the bronchodilatory effect of Beta2-Agonists. Avoid combination
Betahistine: May diminish the therapeutic effect of Beta2-Agonists. Monitor therapy
Cannabinoid-Containing Products: May enhance the tachycardic effect of Sympathomimetics. Exceptions: Cannabidiol. Monitor therapy
Cocaine (Topical): May enhance the hypertensive effect of Sympathomimetics. Management: Consider alternatives to use of this combination when possible. Monitor closely for substantially increased blood pressure or heart rate and for any evidence of myocardial ischemia with concurrent use. Consider therapy modification
Doxofylline: Sympathomimetics may enhance the adverse/toxic effect of Doxofylline. Monitor therapy
Guanethidine: May enhance the arrhythmogenic effect of Sympathomimetics. Guanethidine may enhance the hypertensive effect of Sympathomimetics. Monitor therapy
Haloperidol: QT-prolonging Agents (Indeterminate Risk - Caution) may enhance the QTc-prolonging effect of Haloperidol. Monitor therapy
Linezolid: May enhance the hypertensive effect of Sympathomimetics. Management: Reduce initial doses of sympathomimetic agents, and closely monitor for enhanced pressor response, in patients receiving linezolid. Specific dose adjustment recommendations are not presently available. Consider therapy modification
Loop Diuretics: Beta2-Agonists may enhance the hypokalemic effect of Loop Diuretics. Monitor therapy
Loxapine: Agents to Treat Airway Disease may enhance the adverse/toxic effect of Loxapine. More specifically, the use of Agents to Treat Airway Disease is likely a marker of patients who are likely at a greater risk for experiencing significant bronchospasm from use of inhaled loxapine. Management: This is specific to the Adasuve brand of loxapine, which is an inhaled formulation. This does not apply to non-inhaled formulations of loxapine. Avoid combination
Methacholine: Beta2-Agonists (Short-Acting) may diminish the therapeutic effect of Methacholine. Management: Hold short-acting beta2 agonists for 6 hours before methacholine use. Consider therapy modification
Monoamine Oxidase Inhibitors: May enhance the adverse/toxic effect of Beta2-Agonists. Monitor therapy
QT-prolonging Agents (Highest Risk): QT-prolonging Agents (Indeterminate Risk - Caution) may enhance the QTc-prolonging effect of QT-prolonging Agents (Highest Risk). Management: Monitor for QTc interval prolongation and ventricular arrhythmias when these agents are combined. Patients with additional risk factors for QTc prolongation may be at even higher risk. Monitor therapy
Solriamfetol: Sympathomimetics may enhance the hypertensive effect of Solriamfetol. Monitor therapy
Sympathomimetics: May enhance the adverse/toxic effect of other Sympathomimetics. Monitor therapy
Tedizolid: May enhance the hypertensive effect of Sympathomimetics. Tedizolid may enhance the tachycardic effect of Sympathomimetics. Monitor therapy
Thiazide and Thiazide-Like Diuretics: Beta2-Agonists may enhance the hypokalemic effect of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Tricyclic Antidepressants: May enhance the adverse/toxic effect of Beta2-Agonists. Monitor therapy
Adverse Reactions
>10%:
Central nervous system: Nervousness, restlessness
Endocrine & metabolic: Decreased serum potassium, increased serum glucose
Neuromuscular & skeletal: Tremor
1% to 10%:
Cardiovascular: Hypertension, tachycardia
Central nervous system: Dizziness, drowsiness, headache, insomnia
Dermatologic: Diaphoresis
Gastrointestinal: Dysgeusia, nausea, vomiting, xerostomia
Neuromuscular & skeletal: Muscle cramps, weakness
<1%, postmarketing, and/or case reports: Cardiac arrhythmia, chest pain, hyperglycemia (preterm labor), hypokalemia (preterm labor), hypotension (preterm labor), ischemic heart disease (preterm labor), lactic acidosis (Smith 2019), myocardial infarction (preterm labor), paradoxical bronchospasm, pulmonary edema (preterm labor)
Warnings/Precautions
Concerns related to adverse effects:
- Bronchospasm: Rarely, paradoxical bronchospasm may occur with use of inhaled bronchodilating agents; this should be distinguished from inadequate response.
- Hypersensitivity reactions: Immediate hypersensitivity reactions (urticaria, angioedema, rash, bronchospasm) have been reported.
- Serious effects/fatalities: Do not exceed recommended dose; serious adverse events, including fatalities, have been associated with excessive use of inhaled sympathomimetics.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Disease-related concerns:
- Asthma: Appropriate use: When used as a bronchodilator, optimize anti-inflammatory treatment before initiating maintenance treatment with terbutaline. Do not use as a component of chronic therapy without an anti-inflammatory agent. Only the mildest form of asthma (Step 1 and/or exercise-induced) would not require concurrent use based upon asthma guidelines.
- Cardiovascular disease: Use with caution in patients with cardiovascular disease (arrhythmia or hypertension or HF); beta-agonists may cause elevation in blood pressure, heart rate and result in CNS stimulation/excitation. Beta2-agonists may also increase risk of arrhythmias.
- Diabetes: Use with caution in patients with diabetes mellitus; beta2-agonists may increase serum glucose.
- Glaucoma: Use with caution in patients with glaucoma; may elevate intraocular pressure.
- Hyperthyroidism: Use with caution in hyperthyroidism; may stimulate thyroid activity.
- Hypokalemia: Use with caution in patients with hypokalemia or taking concomitant drugs that cause hypokalemia; beta2-agonists may decrease serum potassium.
- Preterm labor: [US Boxed Warning]: Terbutaline is not FDA approved for and should not be used for prolonged tocolysis (>48 to 72 hours). Use for maintenance tocolysis should not be done in the outpatient setting. Adverse events observed in pregnant women include arrhythmias, increased heart rate, hyperglycemia (transient), hypokalemia, myocardial ischemia, and pulmonary edema. Heart rate may be increased in the fetus and hypoglycemia may occur in the neonate. Oral terbutaline is contraindicated for acute or chronic use in the management of preterm labor.
- Seizures: Use with caution in patients with seizure disorders; beta-agonists may result in CNS stimulation/excitation.
Other warnings/precautions:
- Patient information: Patients must be instructed to seek medical attention in cases where acute symptoms are not relieved or a previous level of response is diminished. The need to increase frequency of use may indicate deterioration of asthma, and treatment must not be delayed.
Monitoring Parameters
Serum potassium, glucose; intake/output; heart rate, blood pressure, respiratory rate; chest pain, shortness of breath; monitor for signs and symptoms of pulmonary edema (when used as a tocolytic); monitor FEV1, peak flow, and/or other pulmonary function tests (when used as bronchodilator). If used for extravasation management, monitor and document extravasation site.
Pregnancy
Pregnancy Considerations
Terbutaline crosses the placenta; umbilical cord concentrations are ~11% to 48% of maternal blood levels.
Terbutaline may affect uterine contractility; use caution if needed to control bronchospasm in pregnant women. Uncontrolled asthma is associated with adverse events in pregnancy (increased risk of perinatal mortality, preeclampsia, preterm birth, low birth weight infants). Poorly controlled asthma or asthma exacerbations may have a greater fetal/maternal risk than what is associated with appropriately used asthma medications. Terbutaline is not one of the preferred agents for the initial treatment of asthma during pregnancy (ACOG 90 2008; GINA 2019; NAEPP 2007).
[US Boxed Warning]: Terbutaline injection has not been approved for and should not be used for prolonged tocolysis (beyond 48 to 72 hours). Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. In particular, terbutaline should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline to pregnant women. In mothers, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema, and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration. Terbutaline has been used in the management of preterm labor. Tocolytics may be used for the short-term (48 hour) prolongation of pregnancy to allow for the administration of antenatal steroids and should not be used prior to fetal viability or when the risks of use to the fetus or mother are greater than the risk of preterm birth (ACOG 171 2016).
Patient Education
What is this drug used for?
- It is used to open the airways in lung diseases where spasm may cause breathing problems.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Fatigue
- Restlessness
- Tremors
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- High blood sugar like confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit.
- Low potassium like muscle pain or weakness, muscle cramps, or an abnormal heartbeat.
- Chest pain
- Fast heartbeat
- Abnormal heartbeat
- Severe anxiety
- Severe dizziness
- Passing out
- Severe headache
- Vision changes
- Seizures
- Difficulty breathing
- Wheezing
- Cough
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.