Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
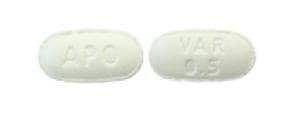
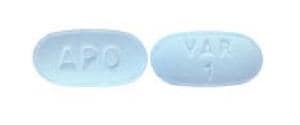
Tablet, Oral:
Chantix: 0.5 mg
Chantix: 1 mg [contains fd&c blue #2 aluminum lake]
Chantix Continuing Month Pak: 1 mg [contains fd&c blue #2 aluminum lake]
Chantix Starting Month Pak: 0.5 mg x 11 & 1 mg x 42
Pharmacology
Mechanism of Action
Partial neuronal α4 β2 nicotinic receptor agonist; prevents nicotine stimulation of mesolimbic dopamine system associated with nicotine addiction. Also binds to 5-HT3 receptor (significance not determined) with moderate affinity. Varenicline stimulates dopamine activity but to a much smaller degree than nicotine does, resulting in decreased craving and withdrawal symptoms.
Pharmacokinetics/Pharmacodynamics
Absorption
Well absorbed; unaffected by food
Metabolism
Minimal (<10% of clearance is through metabolism)
Excretion
Urine (92% as unchanged drug)
Time to Peak
Plasma: ~3 to 4 hours
Half-Life Elimination
~24 hours
Protein Binding
≤20%
Use in Specific Populations
Special Populations: Renal Function Impairment
Moderate renal impairment (CrCl ≥30 to ≤50 mL/minute): Drug exposure increased 1.5-fold
Severe renal impairment (CrCl <30 mL/minute): Drug exposure increased 2.1-fold
ESRD requiring hemodialysis: Drug exposure increased 2.7-fold
Use: Labeled Indications
Smoking cessation: As an aid to smoking cessation treatment.
Contraindications
Serious hypersensitivity reactions or skin reactions to varenicline or any component of the formulation
Dosage and Administration
Dosing: Adult
Smoking cessation: Oral:
Initial:
Days 1 to 3: 0.5 mg once daily.
Days 4 to 7: 0.5 mg twice daily.
Maintenance (day 8 and later): 1 mg twice daily; may consider a temporary or permanent dose reduction if usual dose is not tolerated (Tonstad 2006; manufacturer's labeling).
Duration: Continue maintenance dose for 11 weeks (for a total of 12 weeks of treatment); if the patient successfully quits smoking at the end of 12 weeks, an additional 12-week course may increase likelihood of success (Tonstad 2006).
Approaches to selecting a tobacco quit date: May either choose a fixed quit date (ie, start varenicline, then quit on day 8) or a flexible quit date (ie, start varenicline, then quit between days 8 to 35). Alternatively, a gradual quit date (ie, start varenicline and reduce smoking 50% by week 4, reduce an additional 50% by week 8, and continue reducing with a goal of complete abstinence by week 12) is acceptable (Hajek 2011; Rigotti 2019; manufacturer's labeling).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Smoking cessation: Adolescents ≥17 years: Note: Efficacy has not been established in patients <17 years of age; a randomized, double-blind, placebo-controlled trial including 216 pediatric patients 12 to 16 years of age showed that varenicline did not improve continuous abstinence rates; use is not recommended in pediatric patients ≤16 years of age. In another double-blind, placebo-controlled trial of 157 adolescents and young adults 14 to 19 years of age (mean age: 19.1 ± 1.5 years), the primary efficacy endpoint of end of treatment (week 12) abstinence was the same in both treatment and placebo groups at 8.9%; significant findings in secondary endpoints were observed, including higher weekly self-reported abstinence rates, and patients who achieved 7-day abstinence reported shorter time to achieve 7 days abstinence (39 days compared to 59 days with placebo) (Gray 2019).
Initial: See "Approaches to selecting a tobacco quit date" for additional information.
Days 1 to 3: Oral: 0.5 mg once daily.
Days 4 to 7: Oral: 0.5 mg twice daily.
Maintenance (≥ Day 8): Oral: 1 mg twice daily for 11 weeks; may consider a temporary or permanent dose reduction if usual dose is not tolerated. If patient successfully quits smoking at the end of the 12 weeks, may continue for another 12 weeks to help maintain success. Patients who are motivated to quit and do not succeed in stopping smoking during prior therapy, or who relapse after treatment, should be encouraged to make another attempt with varenicline once factors contributing to the failed attempt have been identified and addressed.
Approaches to selecting a tobacco quit date: May either choose a fixed quit date (ie, start varenicline, then quit on day 8) or a flexible quit date (ie, start varenicline, then quit between days 8 to 35). Alternatively, a gradual quit date (ie, start varenicline and reduce smoking 50% by week 4, reduce an additional 50% by week 8, and continue reducing with a goal of complete abstinence by week 12) is acceptable.
Dosing adjustment for toxicity: Adolescents ≥17 years: Patients who cannot tolerate adverse events may require temporary (or permanent) reduction in dose.
Dosing: Adjustment for Toxicity
Patients who cannot tolerate adverse events may require temporary (or permanent) reduction in dose.
Administration
Oral: Administer after eating and with a full glass of water.
Dietary Considerations
Take after eating and with a full glass of water to decrease gastric upset.
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Varenicline Images
Drug Interactions
Alcohol (Ethyl): Varenicline may enhance the adverse/toxic effect of Alcohol (Ethyl). Specifically, alcohol tolerance may be decreased and the risk for neuropsychiatric adverse effects may be increased. Monitor therapy
Erdafitinib: May increase the serum concentration of OCT2 Substrates. Monitor therapy
Histamine H2 Receptor Antagonists: May increase the serum concentration of Varenicline. Management: Monitor for increased varenicline adverse effects with concomitant use of cimetidine or other H2-antagonists, particularly in patients with severe renal impairment. International product labeling recommendations vary. Consult appropriate labeling. Monitor therapy
Nicotine: Varenicline may enhance the adverse/toxic effect of Nicotine. Monitor therapy
Quinolones: May increase the serum concentration of Varenicline. Management: Monitor for increased varenicline adverse effects with concurrent use of levofloxacin or other quinolone antibiotics, particularly in patients with severe renal impairment. International product labeling recommendations vary. Consult appropriate labeling. Monitor therapy
Tafenoquine: May increase the serum concentration of OCT2 Substrates. Management: Avoid use of OCT2 substrates with tafenoquine, and if the combination cannot be avoided, monitor closely for evidence of toxicity of the OCT2 substrate and consider a reduced dose of the OCT2 substrate according to that substrate's labeling. Consider therapy modification
Trimethoprim: May increase the serum concentration of Varenicline. Management: Monitor for increased varenicline adverse effects with concomitant use of trimethoprim, particularly in patients with severe renal impairment. International product labeling recommendations vary. Consult appropriate labeling. Monitor therapy
Adverse Reactions
>10%:
Central nervous system: Headache (12% to 19%), insomnia (9% to 19%), abnormal dreams (8% to 13%), irritability (11%), suicidal ideation (11%), depression (4% to 11%)
Gastrointestinal: Nausea (16% to 40%), vomiting (5% to 11%)
1% to 10%:
Cardiovascular: Angina pectoris (4%), chest pain (3%), peripheral edema (2%), myocardial infarction (≤1%)
Central nervous system: Anxiety (8%), malaise (7%), agitation (5% to 7%), sleep disorder (3% to 5%), tension (4%), drowsiness (3%), hostility (2% to 3%), lethargy (1% to 2%), nightmares (1% to 2%)
Dermatologic: Skin rash (3%)
Gastrointestinal: Flatulence (6% to 9%), constipation (5% to 8%), dysgeusia (5% to 8%), abdominal pain (7%), diarrhea (6%), xerostomia (6%), dyspepsia (5%), increased appetite (3% to 4%), anorexia (≤2%), decreased appetite (≤2%), gastroesophageal reflux disease (1%)
Respiratory: Upper respiratory tract infection (5% to 7%), dyspnea (2%), rhinorrhea (≤1%)
<1%, postmarketing, and/or case reports: Abnormal hepatic function tests, abnormality in thinking, abnormal urinalysis, accidental injury, acne vulgaris, acute coronary syndrome, acute renal failure, aggressive behavior, allergic rhinitis, altered sense of smell, amnesia, anemia, angioedema, arthralgia, asthma, atrial fibrillation, back pain, behavioral changes, Bell palsy, blurred vision, bradycardia, cardiac arrhythmia, cardiac flutter, cataract (subcapsular), cerebrovascular accident, chills, conjunctivitis, cor pulmonale, coronary artery disease, deafness, decreased libido, decreased mental acuity, decreased visual acuity, delusions, diabetes mellitus, difficulty thinking, disorientation, dissociative disorder, dizziness, dysarthria, dysphagia, ECG abnormality, eczema, edema, elevation in serum levels of skeletal-muscle enzymes, emotional disturbance, emotional lability, enterocolitis, epistaxis, equilibrium disturbance, erectile dysfunction, eructation, erythema, erythema multiforme, esophagitis, euphoria, eye irritation, eye pain, fever, flu-like symptoms, flushing, gallbladder disease, gastric ulcer, gastritis, gastrointestinal hemorrhage, hallucination, homicidal ideation, hyperglycemia, hyperhidrosis, hyperlipidemia, hypersensitivity reaction, hypoglycemia, hypokalemia, intestinal obstruction, lack of concentration, leukocytosis, loss of consciousness, lymphadenopathy, mania, Meniere disease, menstrual disease, migraine, multiple sclerosis, muscle cramps, musculoskeletal pain, myalgia, myositis, nephrolithiasis, nocturia, nocturnal amblyopia, nystagmus, ophthalmic vascular disease, oral mucosa ulcer, osteoporosis, palpitations, pancreatitis, panic, paranoia, photophobia, pleurisy, pollakiuria, polyuria, psoriasis, psychomotor agitation, psychomotor retardation, psychosis, pulmonary embolism, respiratory tract disease, restless leg syndrome, seizure, sensory disturbance, sexual difficulty, skin photosensitivity, somnambulism, splenomegaly, Stevens-Johnson syndrome, syncope, tachycardia, thrombocytopenia, thrombosis, thyroid disease, tinnitus, toothache, transient blindness, transient ischemic attacks, tremor, upper respiratory tract inflammation, urethral disease, urinary retention, urine abnormality, urticaria, ventricular premature contractions, vertigo, visual field defect, vitreous opacity, weight gain, xeroderma, xerophthalmia
Warnings/Precautions
Concerns related to adverse effects:
- CNS depression: May cause CNS depression, which may impair physical or mental abilities; patients must be cautioned about performing tasks which require mental alertness (eg, operating machinery or driving). There have been postmarketing reports of traffic accidents, near-miss incidents in traffic, or other accidental injuries in patients taking varenicline.
- Hypersensitivity reactions: Postmarketing reports of hypersensitivity reactions (including angioedema) and rare cases of serious skin reactions (including Stevens-Johnson syndrome and erythema multiforme) have been reported. Patients should be instructed to discontinue use and contact healthcare provider if signs/symptoms occur.
- Nausea: Dose-dependent nausea may occur; both transient and persistent nausea has been reported. Dosage reduction may be considered for intolerable nausea.
- Neuropsychiatric effects: Postmarketing cases of serious neuropsychiatric events (including depression, suicidal thoughts, and suicide) have been reported in patients with or without preexisting psychiatric disease; some cases may have been complicated by symptoms of nicotine withdrawal following smoking cessation. Subsequent controlled trials in patients with or without psychiatric disorders; however, have not identified significant differences in neuropsychiatric effects for patients taking varenicline, bupropion, nicotine patches, or placebo (Anthenelli 2013; Anthenelli 2016; Gibbons 2013; Thomas 2015). Monitor all patients for behavioral changes and psychiatric symptoms (eg, agitation, depression, suicidal behavior, suicidal ideation); inform patients to discontinue treatment and contact their health care provider immediately if they experience any behavioral and/or mood changes. Of postmarketing cases, many resolved following therapy discontinuation.
- Somnambulism: Cases of somnambulism, involving harmful behavior to self, others or property, have been reported. Discontinue treatment if somnambulism occurs.
Disease-related concerns:
- Cardiovascular events: Treatment may increase risk of cardiovascular events. A meta-analysis of 15 clinical trials, including a placebo-controlled trial in patients with stable cardiovascular disease, showed an increased incidence of major cardiovascular events (combined outcome of cardiovascular-related death, nonfatal MI, nonfatal stroke) in patients using varenicline compared with placebo. Cardiovascular events were uncommon in both the varenicline and placebo groups. These findings did not reach statistical significance, although data was consistent. Events occurred primarily in patients with known cardiovascular disease. The meta-analysis also showed a lower incidence of all-cause and cardiovascular mortality in varenicline-treated patients, although this was not statistically significant either. Patients should be instructed to contact their health care provider if cardiovascular symptoms occur.
- Renal impairment: Use with caution in patients with renal impairment; dosage adjustment required with severe impairment.
- Seizures: Seizures have been reported in patients with or without a history of seizures. Seizures generally occurred within the first month of therapy. Consider the risks against the benefits before initiating in patients with a history of seizures or other factors that can lower the seizure threshold; discontinue use if seizures occur during therapy.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Monitoring Parameters
Monitor for behavioral changes and psychiatric symptoms (eg, agitation, depression, suicidal behavior, suicidal ideation).
Pregnancy
Pregnancy Considerations
Information related to the use of varenicline in pregnancy is limited (Harrison-Woolrych 2013; Kaplan 2014; Richardson 2017).
Nicotine exposure is associated with adverse events to both the mother and fetus. All pregnant females should be encouraged to stop smoking. However, data is insufficient to recommend varenicline for smoking cessation during pregnancy (ACOG 721 2017).
Patient Education
What is this drug used for?
- It is used to help you stop smoking.
Frequently reported side effects of this drug
- Passing gas
- Trouble sleeping
- Headache
- Nightmares
- Constipation
- Dry mouth
- Abdominal pain
- Change in taste
- Common cold symptoms
- Loss of strength and energy
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Depression like thoughts of suicide, anxiety, emotional instability, or confusion.
- Sensing things that seem real but are not
- Psychosis
- Anxiety
- Agitation
- Mood changes
- Behavioral changes
- Swelling in your throat
- Severe cerebrovascular disease like change in strength on one side is greater than the other, difficulty speaking or thinking, change in balance, or vision changes.
- Heart attack like chest pain; pain in arms, back, neck, jaw, or abdomen; shortness of breath; cold sweats; severe dizziness; passing out; or severe nausea or vomiting.
- Shortness of breath
- Severe nausea
- Vomiting
- Seizures
- Confusion
- Sleepwalking
- Stevens-Johnson syndrome/toxic epidermal necrolysis like red, swollen, blistered, or peeling skin (with or without fever); red or irritated eyes; or sores in mouth, throat, nose, or eyes.
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.