Boxed Warning
Risk from concomitant use with opioids:
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
KlonoPIN: 0.5 mg [scored; contains fd&c yellow #6 aluminum lake]
KlonoPIN: 1 mg [contains fd&c blue #1 aluminum lake, fd&c blue #2 aluminum lake]
KlonoPIN: 2 mg
Generic: 0.5 mg, 1 mg, 2 mg
Tablet Disintegrating, Oral:
Generic: 0.125 mg, 0.25 mg, 0.5 mg, 1 mg, 2 mg
Pharmacology
Mechanism of Action
The exact mechanism is unknown, but believed to be related to its ability to enhance the activity of GABA; suppresses the spike-and-wave discharge in absence seizures by depressing nerve transmission in the motor cortex.
Pharmacokinetics/Pharmacodynamics
Absorption
Rapidly and completely absorbed
Distribution
Children: Vd: 1.5 to 3 L/kg (Walson 1996); Adults: Vd: 1.5 to 6.4 L/kg (Walson 1996)
Metabolism
Extensively hepatic via glucuronide and sulfate conjugation; undergoes nitroreduction to 7-aminoclonazepam, followed by acetylation to 7-acetamidoclonazepam; nitroreduction and acetylation are via CYP3A4; metabolites undergo glucuronide and sulfate conjugation (Patsalos 2018).
Excretion
Urine (<2% as unchanged drug); metabolites excreted as glucuronide or sulfate conjugates
Onset of Action
~20 to 40 minutes (Hanson 1972)
Time to Peak
Serum: 1 to 4 hours
Duration of Action
Infants and young children: 6 to 8 hours (Hanson 1972); Adults: ≤12 hours (Hanson 1972)
Half-Life Elimination
Neonates: 22 to 81 hours (Patsalos 2018); Children: 22 to 33 hours (Walson 1996); Adults: 17 to 60 hours (Walson 1996).
Protein Binding
~85%
Use: Labeled Indications
Panic disorder: Treatment of panic disorder, with or without agoraphobia.
Seizure disorders: Mono- or adjunctive therapy in the treatment of the Lennox-Gastaut syndrome (petit mal variant), akinetic, and myoclonic seizures; absence seizures (petit mal) unresponsive to succinimides.
Use: Off Label
Bipolar disorder, manic or mixed episodesbyes
Data from a meta-analysis of 5 randomized, controlled trials supports the use of clonazepam in the treatment of acute bipolar mania Curtin 2004. Additional trials may be necessary to further define the role of clonazepam in this condition.
APA guidelines for the treatment of patients with bipolar disorder suggest that the use of benzodiazepines, such as clonazepam, in sedative doses may be useful short-term as adjunctive therapy when used with traditional mood-stabilizing agents. CANMAT guidelines for the management of patients with bipolar disorder recommend against the use of benzodiazepines as monotherapy, but suggest they may be useful adjunctive therapy for the sedation of acutely agitated patients. Similarly, WFSBP guidelines for the treatment of acute mania in bipolar disorder recommend benzodiazepines as adjunctive therapy to target anxiety and insomnia in patients taking mood-stabilizing agents.
Burning mouth syndromec
Data from a prospective open-label study in patients with mouth burning without oral mucosal lesions suggest that clonazepam may be helpful in patients with this condition Grushka 1998. Additional data from a double-blind, randomized, multicenter, parallel group study using topical (buccal administration) clonazepam demonstrated improvement in pain intensity Gremeau-Richard 2004. In a systematic review, clonazepam is a therapeutic option in the treatment of this condition; however, use should be limited due to the risk of benzodiazepine dependence Buchanan 2008. Additional trials are necessary to further define the role of clonazepam for the treatment of this condition.
Essential tremorcyes
Data from a limited number of patients studied (case series) suggest that clonazepam may be beneficial in reducing the frequency of essential tremor Biary 1987. On the contrary, data from a small randomized, double-blind, placebo-controlled trial demonstrated that clonazepam was not an effective treatment Thompson 1984. Additional data may be necessary to further define the role of clonazepam in this condition.
Based on the American Academy of Neurology guidelines for the treatment of essential tremor, clonazepam is possibly effective for the treatment of this condition.
Rapid eye movement (REM) sleep behavior disorderyes
Based on the American Academy of Sleep Medicine (AASM) Best Practice Guide for the Treatment of REM Sleep Behavior Disorder (RBD), clonazepam is an effective and recommended treatment option for patients with this condition; however, it should be used with caution in patients with dementia, gait disorders, or concomitant obstructive sleep apnea AASM [Aurora 2010].
Restless legs syndromec
Data from a limited number of patients in small controlled trials suggest that clonazepam may be beneficial for the treatment of periodic limb movements and poor sleep associated with restless legs syndrome Montagna 1984, Peled 1987, Saletu 2001, Schenck 1996. Additional data may be necessary to further define the role of clonazepam in this condition.
Based on the updated
Tardive dyskinesiacyes
Data from a limited number of patients in a double-blind, randomized, placebo-controlled crossover trial suggests that clonazepam may be beneficial for the treatment of tardive dyskinesia Thaker 1990. Additional data may be necessary to further define the role of clonazepam in this condition.
Based on the American Academy of Neurology guideline for the treatment of tardive syndromes, clonazepam given for tardive dyskinesia is probably effective in decreasing tardive dyskinesia symptoms in the short-term (approximately 3 months) and is suggested for the short-term treatment of tardive dyskinesia.
Tic disordersc
Data from a limited number of patients studied in a single-blind and two retrospective studies suggest that clonazepam may be beneficial for multifocal tic disorder or Tourette disorder. Additional data may be necessary to further define the role of clonazepam in these conditions.
Contraindications
Hypersensitivity to clonazepam, other benzodiazepines, or any component of the formulation; significant liver disease; acute narrow-angle glaucoma
Canadian labeling: Additional contraindications (not in US labeling): Severe respiratory insufficiency; sleep apnea syndrome; myasthenia gravis
Dosage and Administration
Dosing: Adult
Panic disorder: Oral: 0.25 mg twice daily; increase in increments of 0.125 to 0.25 mg twice daily every 3 days; target dose: 1 mg daily (maximum: 4 mg/day).
Discontinuation of treatment: To discontinue, treatment should be withdrawn gradually. Decrease dose by 0.125 mg twice daily every 3 days until medication is completely withdrawn.
Seizure disorders: Oral:
Initial daily dose not to exceed 1.5 mg given in 3 divided doses; may increase by 0.5 to 1 mg every third day until seizures are controlled or adverse effects seen (maximum: 20 mg/day).
Usual maintenance dose: 2 to 8 mg daily in 1 to 2 divided doses (Brodie 1997); do not exceed 20 mg/day.
Bipolar disorder, mixed or manic episodes (off-label use): Oral: 2 to 8 mg daily, in 2 to 4 divided doses; total daily doses as high as 16 mg have been studied (Bottai 1995; Chouinard 1983; Clark 1997; Edwards 1991; WFSBP [Grunze 2009]).
Burning mouth syndrome (off-label use):
Oral: Initial: 0.25 at bedtime for 1 week; increase dose by ≤0.25 mg every week; maximum dose: 3 mg daily in 3 divided doses. Note: Use should be limited (Buchanan 2008; Grushka 1998).
Topical: May administer topically with 1 mg 3 times daily (after each meal). Note: Patient should be instructed to suck on the tablet, retain saliva in mouth near the pain sites without swallowing for 3 minutes, and then expectorate saliva (Gremeau-Richard 2004).
Essential tremor (off-label use): Oral: Initial: 0.5 mg at bedtime; increase dose by 0.5 mg every 3 to 4 days; maximum dose: 6 mg daily (Biary 1987; Thompson 1984; Zesiewicz 2005; Zesiewicz 2011).
REM sleep behavior disorder (off-label use): 0.25 to 2 mg 30 minutes prior to bedtime (maximum: 4 mg 30 minutes prior to bedtime). Note: Use with caution in patients with dementia, gait disorders, or obstructive sleep apnea (Aurora 2010).
Restless leg syndrome (off-label use): Oral: Initial: 1 mg 30 minutes prior to bedtime; increase dose by 0.5 to 1 mg at weekly intervals. Doses up to 2 mg once daily have been used in clinical trials (Montagna 1984; Peled 1987; Saletu 2001). Additional data may be necessary to further define the role of clonazepam in the treatment of this condition.
Tardive dyskinesia (off-label use): Oral: Initial: 1 mg/day; adjust dosage based on response and tolerability by 1 mg/day every 3 to 4 days up to a maximum dose of 4.5 mg/day (Thaker 1990).
Tic disorders (off-label use): Oral: Initial: 0.5 mg at bedtime; adjust dose by 0.5 mg every 2 weeks based on response and tolerability. Dosing range in clinical studies was 1 to 12 mg/day (Merikangas 1985; Troung 1988).
Dosing: Geriatric
Refer to adult dosing. Initiate with low doses and observe closely.
Dosing: Pediatric
Note: If necessary to discontinue clonazepam therapy, drug should be withdrawn gradually.
Neuroirritability, agitation (palliative care): Limited data available: Infants, Children, and Adolescents: Oral:
Patient weight:
<30 kg: Initial: 0.01 to 0.03 mg/kg/day in divided doses up to 3 to 4 times daily; increase dose to desired effect up to a maximum daily dose: 0.2 mg/kg/day in 3 divided doses (Kliegman 2017; Wustoff 2007)
≥30 kg: Initial: ≤0.25 mg/dose 3 times daily; may increase by 0.5 to 1 mg/day every 3 days up to maintenance dose range: 0.05 to 0.2 mg/kg/day up to maximum daily dose: 20 mg/day (Kliegman 2017)
Seizure disorders:
Infants and Children <10 years or ≤30 kg: Oral:
Initial: 0.01 to 0.03 mg/kg/day in 2 to 3 divided doses; maximum initial daily dose: 0.05 mg/kg/day; increase by ≤0.25 to 0.5 mg every third day until seizures are controlled or adverse effects observed
Maintenance dose: 0.1 to 0.2 mg/kg/day in 3 divided doses; maximum daily dose: 0.2 mg/kg/day
Children ≥10 years or >30 kg and Adolescents: Oral:
Initial: 0.01 to 0.05 mg/kg/day in 2 or 3 divided doses; maximum initial dose: 0.5 mg/dose 3 times daily; may increase dose by 25% or by 0.5 to 1 mg every 3 to 7 days until seizures are controlled or adverse effects observed (Kliegman 2017)
Maintenance dose range: 0.05 to 0.2 mg/kg/day in 2 to 3 divided doses; maximum daily dose: 20 mg/day (Kliegman 2017)
Panic disorder: Adolescents ≥18 years: Oral: Initial: 0.25 mg twice daily; increase in increments of 0.125 to 0.25 mg twice daily every 3 days; target dose: 1 mg/day in divided doses; some patients may require higher doses up to a maximum daily dose: 4 mg/day. To discontinue, treatment should be withdrawn gradually; decrease dose by 0.125 mg twice daily every 3 days until medication is completely withdrawn.
Extemporaneously Prepared
A 0.1 mg/mL oral suspension may be made with tablets and one of three different vehicles (cherry syrup; a 1:1 mixture of Ora-Sweet® and Ora-Plus®; or a 1:1 mixture of Ora-Sweet® SF and Ora-Plus®). Crush six 2 mg tablets in a mortar and reduce to a fine powder. Add 10 mL of the chosen vehicle and mix to a uniform paste; mix while adding the vehicle in incremental proportions to almost 120 mL; transfer to a calibrated bottle, rinse mortar with vehicle, and add quantity of vehicle sufficient to make 120 mL. Label “shake well” and “protect from light”. Stable for 60 days when stored in amber prescription bottles in the dark at room temperature or refrigerated.
Allen LV Jr and Erickson MA 3rd, "Stability of Acetazolamide, Allopurinol, Azathioprine, Clonazepam, and Flucytosine in Extemporaneously Compounded Oral Liquids," Am J Health Syst Pharm 1996, 53(16):1944-9.8862208
Administration
To reduce somnolence, administration of one dose at bedtime may be desirable.
Orally-disintegrating tablet: Open pouch and peel back foil on the blister; do not push tablet through foil. Use dry hands to remove tablet and place in mouth. May be swallowed with or without water. Use immediately after removing from package.
Tablet: Swallow whole with water.
Storage
Tablets: Store at 20°C to 25°C (68°F to 77°F).
Orally disintegrating tablets: Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)
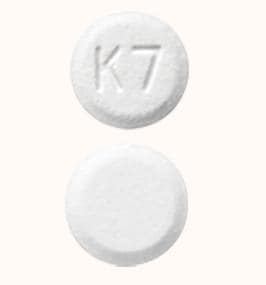
ClonazePAM Images
Drug Interactions
Alcohol (Ethyl): CNS Depressants may enhance the CNS depressant effect of Alcohol (Ethyl). Monitor therapy
Alizapride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Aprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Azelastine (Nasal): CNS Depressants may enhance the CNS depressant effect of Azelastine (Nasal). Avoid combination
Blonanserin: CNS Depressants may enhance the CNS depressant effect of Blonanserin. Consider therapy modification
Bosentan: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Brexanolone: CNS Depressants may enhance the CNS depressant effect of Brexanolone. Monitor therapy
Brimonidine (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Bromopride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Bromperidol: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
Buprenorphine: CNS Depressants may enhance the CNS depressant effect of Buprenorphine. Management: Consider reduced doses of other CNS depressants, and avoiding such drugs in patients at high risk of buprenorphine overuse/self-injection. Initiate buprenorphine at lower doses in patients already receiving CNS depressants. Consider therapy modification
Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Cannabis: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Chlormethiazole: May enhance the CNS depressant effect of CNS Depressants. Management: Monitor closely for evidence of excessive CNS depression. The chlormethiazole labeling states that an appropriately reduced dose should be used if such a combination must be used. Consider therapy modification
Chlorphenesin Carbamate: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
Clofazimine: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CloZAPine: Benzodiazepines may enhance the adverse/toxic effect of CloZAPine. Management: Consider decreasing the dose of (or possibly discontinuing) benzodiazepines prior to initiating clozapine. Consider therapy modification
CNS Depressants: May enhance the adverse/toxic effect of other CNS Depressants. Monitor therapy
Cobicistat: May increase the serum concentration of ClonazePAM. Monitor therapy
Conivaptan: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Cosyntropin: May enhance the hepatotoxic effect of ClonazePAM. Monitor therapy
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May increase the metabolism of CYP3A4 Substrates (High risk with Inducers). Management: Consider an alternative for one of the interacting drugs. Some combinations may be specifically contraindicated. Consult appropriate manufacturer labeling. Consider therapy modification
CYP3A4 Inhibitors (Moderate): May decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
CYP3A4 Inhibitors (Strong): May decrease the metabolism of CYP3A4 Substrates (High risk with Inhibitors). Consider therapy modification
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Dimethindene (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Doxylamine: May enhance the CNS depressant effect of CNS Depressants. Management: The manufacturer of Diclegis (doxylamine/pyridoxine), intended for use in pregnancy, specifically states that use with other CNS depressants is not recommended. Monitor therapy
Dronabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Droperidol: May enhance the CNS depressant effect of CNS Depressants. Management: Consider dose reductions of droperidol or of other CNS agents (eg, opioids, barbiturates) with concomitant use. Exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Consider therapy modification
Duvelisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Enzalutamide: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Concurrent use of enzalutamide with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP3A4 substrate should be performed with caution and close monitoring. Consider therapy modification
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Erdafitinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Esketamine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Flunitrazepam: CNS Depressants may enhance the CNS depressant effect of Flunitrazepam. Consider therapy modification
Fosaprepitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fosnetupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Fosphenytoin: May decrease the serum concentration of ClonazePAM. Clonazepam may also alter concentrations of Phenytoin (active metabolite of Fosphenytoin). Monitor therapy
Fusidic Acid (Systemic): May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
HYDROcodone: CNS Depressants may enhance the CNS depressant effect of HYDROcodone. Management: Avoid concomitant use of hydrocodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
HydrOXYzine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Idelalisib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Avoid combination
Ivosidenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Kava Kava: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
Larotrectinib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Lemborexant: May enhance the CNS depressant effect of CNS Depressants. Management: Dosage adjustments of lemborexant and of concomitant CNS depressants may be necessary when administered together because of potentially additive CNS depressant effects. Close monitoring for CNS depressant effects is necessary. Consider therapy modification
Lofexidine: May enhance the CNS depressant effect of CNS Depressants. Management: Drugs listed as exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Monitor therapy
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
Magnesium Sulfate: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Melatonin: May enhance the sedative effect of Benzodiazepines. Monitor therapy
Methadone: Benzodiazepines may enhance the CNS depressant effect of Methadone. Management: Clinicians should generally avoid concurrent use of methadone and benzodiazepines when possible; any combined use should be undertaken with extra caution. Consider therapy modification
Methotrimeprazine: CNS Depressants may enhance the CNS depressant effect of Methotrimeprazine. Methotrimeprazine may enhance the CNS depressant effect of CNS Depressants. Management: Reduce adult dose of CNS depressant agents by 50% with initiation of concomitant methotrimeprazine therapy. Further CNS depressant dosage adjustments should be initiated only after clinically effective methotrimeprazine dose is established. Consider therapy modification
MetyroSINE: CNS Depressants may enhance the sedative effect of MetyroSINE. Monitor therapy
MiFEPRIStone: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Minimize doses of CYP3A4 substrates, and monitor for increased concentrations/toxicity, during and 2 weeks following treatment with mifepristone. Avoid cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, and tacrolimus. Consider therapy modification
Minocycline (Systemic): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Mitotane: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Doses of CYP3A4 substrates may need to be adjusted substantially when used in patients being treated with mitotane. Consider therapy modification
Nabilone: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Netupitant: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
OLANZapine: May enhance the adverse/toxic effect of Benzodiazepines. Management: Avoid concomitant use of parenteral benzodiazepines and IM olanzapine due to risks of additive adverse events (e.g., cardiorespiratory depression). Olanzapine prescribing information provides no specific recommendations regarding oral administration. Avoid combination
Opioid Agonists: CNS Depressants may enhance the CNS depressant effect of Opioid Agonists. Management: Avoid concomitant use of opioid agonists and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Orphenadrine: CNS Depressants may enhance the CNS depressant effect of Orphenadrine. Avoid combination
Oxomemazine: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
OxyCODONE: CNS Depressants may enhance the CNS depressant effect of OxyCODONE. Management: Avoid concomitant use of oxycodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Palbociclib: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Paraldehyde: CNS Depressants may enhance the CNS depressant effect of Paraldehyde. Avoid combination
Perampanel: May enhance the CNS depressant effect of CNS Depressants. Management: Patients taking perampanel with any other drug that has CNS depressant activities should avoid complex and high-risk activities, particularly those such as driving that require alertness and coordination, until they have experience using the combination. Consider therapy modification
Phenytoin: May decrease the serum concentration of ClonazePAM. Clonazepam may also alter concentrations of Phenytoin. Monitor therapy
Piribedil: CNS Depressants may enhance the CNS depressant effect of Piribedil. Monitor therapy
Pramipexole: CNS Depressants may enhance the sedative effect of Pramipexole. Monitor therapy
ROPINIRole: CNS Depressants may enhance the sedative effect of ROPINIRole. Monitor therapy
Rotigotine: CNS Depressants may enhance the sedative effect of Rotigotine. Monitor therapy
Rufinamide: May enhance the adverse/toxic effect of CNS Depressants. Specifically, sleepiness and dizziness may be enhanced. Monitor therapy
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Selective Serotonin Reuptake Inhibitors: CNS Depressants may enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Specifically, the risk of psychomotor impairment may be enhanced. Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Simeprevir: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Monitor therapy
Sodium Oxybate: Benzodiazepines may enhance the CNS depressant effect of Sodium Oxybate. Avoid combination
Stiripentol: May increase the serum concentration of CYP3A4 Substrates (High risk with Inhibitors). Management: Use of stiripentol with CYP3A4 substrates that are considered to have a narrow therapeutic index should be avoided due to the increased risk for adverse effects and toxicity. Any CYP3A4 substrate used with stiripentol requires closer monitoring. Consider therapy modification
Suvorexant: CNS Depressants may enhance the CNS depressant effect of Suvorexant. Management: Dose reduction of suvorexant and/or any other CNS depressant may be necessary. Use of suvorexant with alcohol is not recommended, and the use of suvorexant with any other drug to treat insomnia is not recommended. Consider therapy modification
Tapentadol: May enhance the CNS depressant effect of CNS Depressants. Management: Avoid concomitant use of tapentadol and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Teduglutide: May increase the serum concentration of Benzodiazepines. Monitor therapy
Tetrahydrocannabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Tetrahydrocannabinol and Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Thalidomide: CNS Depressants may enhance the CNS depressant effect of Thalidomide. Avoid combination
Theophylline Derivatives: May diminish the therapeutic effect of Benzodiazepines. Consider therapy modification
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Trimeprazine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Vigabatrin: May enhance the CNS depressant effect of ClonazePAM. Vigabatrin may increase the serum concentration of ClonazePAM. Monitor therapy
Yohimbine: May diminish the therapeutic effect of Antianxiety Agents. Monitor therapy
Zolpidem: CNS Depressants may enhance the CNS depressant effect of Zolpidem. Management: Reduce the Intermezzo brand sublingual zolpidem adult dose to 1.75 mg for men who are also receiving other CNS depressants. No such dose change is recommended for women. Avoid use with other CNS depressants at bedtime; avoid use with alcohol. Consider therapy modification
Adverse Reactions
Reactions reported in patients with seizure disorder, unless otherwise noted. Frequency not always defined.
>10%: Central nervous system: Drowsiness (seizure disorder: ~50%; panic disorder: 26% to 50%), ataxia (seizure disorder: ~30%; panic disorder: 1% to 9%), behavioral problems (seizure disorder: ~25%), dizziness (panic disorder: 5% to 12%)
1% to 10%:
Central nervous system: Fatigue (panic disorder: 6% to 9%), depression (panic disorder: 6% to 8%), memory impairment (panic disorder: 4% to 5%), nervousness (panic disorder: 3% to 4%), dysarthria (panic disorder: ≤4%), reduced intellectual ability (panic disorder: ≤4%), emotional lability (panic disorder: 2%), confusion (panic disorder: ≤2%), delayed ejaculation (panic disorder ≤2%)
Endocrine & metabolic: Decreased libido (panic disorder: ≤3%)
Gastrointestinal: Constipation (panic disorder: 3% to 5%), decreased appetite (panic disorder: 3%), abdominal pain (panic disorder: 2%)
Genitourinary: Dysmenorrhea (panic disorder: 3% to 6%), vaginitis (panic disorder: 2% to 4%), impotence (panic disorder: ≤3%), urinary tract infection (panic disorder: ≤2%), urinary frequency (panic disorder: 1% to 2%)
Hypersensitivity: Hypersensitivity (panic disorder: 2% to 4%)
Neuromuscular & skeletal: Myalgia (panic disorder: 2% to 4%)
Ophthalmic: Blurred vision (panic disorder: 2% to 3%)
Respiratory: Upper respiratory tract infection (panic disorder: 6% to 10%), sinusitis (panic disorder: 4% to 8%), influenza (panic disorder: 4% to 5%), cough (panic disorder: ≤4%), rhinitis (panic disorder: 2% to 4%), pharyngitis (panic disorder: 2% to 3%), bronchitis (panic disorder: 2%)
Frequency not defined:
Cardiovascular: Edema (ankle or facial), palpitations
Central nervous system: Amnesia, aphonia, choreiform movements, coma, glassy-eyed appearance, hallucination, headache, hemiparesis, hypotonia, hysteria, insomnia, myasthenia, psychosis, slurred speech, vertigo
Dermatologic: Alopecia, skin rash
Endocrine & metabolic: Dehydration, hirsutism, increased libido, weight gain, weight loss
Gastrointestinal: Anorexia, coated tongue, diarrhea, encopresis, gastritis, gingival pain, increased appetite, nausea, xerostomia
Genitourinary: Dysuria, nocturia, urinary incontinence, urinary retention
Hematologic & oncologic: Anemia, eosinophilia, leukopenia, lymphadenopathy, thrombocytopenia
Hepatic: Hepatomegaly, increased serum alkaline phosphatase (transient), increased serum transaminases (transient)
Neuromuscular & skeletal: Dysdiadochokinesia, tremor
Ophthalmic: Abnormal eye movements, diplopia, nystagmus
Respiratory: Chest congestion, dyspnea, respiratory depression, rhinorrhea, upper respiratory complaint (hypersecretion)
Miscellaneous: Fever, paradoxical reactions (including aggressive behavior, agitation, anxiety excitability, hostility, irritability, nervousness, nightmares, sleep disturbance, vivid dreams), physical health deterioration
<1%, postmarketing, and/or case reports (any indication): Abdominal distress, abnormal behavior (increased oppositional behavior), accidental injury, acne flare, ageusia, aggressive behavior, alcohol intoxication, anxiety, apathy, arthralgia, back pain, bladder dysfunction, bone fracture, burn, burning sensation of skin, candidiasis, cellulitis, chest pain, contact dermatitis, cystitis, depersonalization, dermal hemorrhage, dermatological reaction, disinhibition (organic), dyspepsia, ejaculatory disorder, epistaxis, exacerbation of asthma, excitement, excoriation, eye irritation, falling, flatulence, flushing, foot pain, frequent bowel movements, fungal infection, gastric distress, gout, heartburn, heavy headedness, hemorrhoids, herpes simplex infection, hoarseness, hordeolum, hyperactivity, hypertonia, hypoesthesia, hunger, illusion, increased dream activity, increased thirst, infectious mononucleosis, irregular menses, irritability, jaw pain, knee effusion, knee pain, lack of concentration, leg pain, leg thrombophlebitis, local inflammation, lower back pain, malaise, mastalgia, migraine, motion sickness, orthostatic hypotension, otalgia, otitis, pain, paresis, paresthesia, pedal edema, pelvic pain, periorbital edema, pleurisy, pneumonia, polyuria, pruritus, pustular rash, shivering, shoulder pain, sialorrhea, sleep disorder, slowed reaction time, sneezing, sprain, strain, streptococcal infection, suicidal ideation, suicidal tendencies, tendonitis, tongue edema, toothache, twitching, twitching of eye, urinary tract hemorrhage, urine discoloration, viral infection, visual disturbance, visual field defect, withdrawal syndrome, xeroderma, xerophthalmia, yawning
Warnings/Precautions
Concerns related to adverse effects:
- Anterograde amnesia: Benzodiazepines have been associated with anterograde amnesia (Nelson 1999).
- CNS depression: May cause CNS depression, which may impair physical or mental abilities; patients must be cautioned about performing tasks which require mental alertness (eg, operating machinery or driving); increased risk may occur with the use of multiple anticonvulsants.
- Paradoxical reactions: Paradoxical reactions, including hyperactive or aggressive behavior, have been reported with benzodiazepines; risk may be increased in adolescent/pediatric patients, geriatric patients, or patients with a history of alcohol use disorder or psychiatric/personality disorders (Mancuso 2004).
- Sleep-related activities: Hazardous sleep-related activities such as sleep-driving, cooking and eating food, and making phone calls while asleep have been noted with benzodiazepines (Dolder 2008).
- Suicidal ideation: Pooled analysis of trials involving various antiepileptics (regardless of indication) showed an increased risk of suicidal thoughts/behavior (incidence rate: 0.43% treated patients compared to 0.24% of patients receiving placebo); risk observed as early as 1 week after initiation and continued through duration of trials (most trials ≤24 weeks). Monitor all patients for notable changes in behavior that might indicate suicidal thoughts or depression; notify health care provider immediately if symptoms occur.
Disease-related concerns:
- Depression: Use caution in patients with depression, particularly if suicidal risk may be present.
- Drug abuse: Use with caution in patients with a history of drug abuse or acute alcoholism; potential for drug dependency exists. Tolerance, psychological and physical dependence may occur with prolonged use.
- Glaucoma: May be used in patients with open angle glaucoma who are receiving appropriate therapy; contraindicated in acute narrow angle glaucoma.
- Hepatic impairment: Use with caution in patients with hepatic impairment; accumulation likely to occur. Contraindicated in patients with significant hepatic impairment.
- Porphyria: Use with caution in patients with porphyria; may have a porphyrogenic effect.
- Renal impairment: Use with caution in patients with renal impairment; clonazepam metabolites are renally eliminated.
- Respiratory disease: Clonazepam may cause respiratory depression and may produce an increase in salivation; use with caution in patients with compromised respiratory function (eg, chronic obstructive pulmonary disease, sleep apnea) and in patients who have difficulty handling secretions.
Concurrent drug therapy issues:
- Concomitant use with opioids: [US Boxed Warning]: Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation.
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Debilitated patients: Use with caution in debilitated patients.
- Elderly patients: Elderly patients may be at an increased risk of death with use; risk has been found highest within the first 4 months of use in elderly dementia patients (Jennum 2015; Saarelainen 2018).
- Fall risk: Use with extreme caution in patients who are at risk of falls; benzodiazepines have been associated with falls and traumatic injury (Nelson 1999).
Other warnings/precautions:
- Appropriate use: Does not have analgesic, antidepressant, or antipsychotic properties. Worsening of seizures may occur when added to patients with multiple seizure types. Loss of anticonvulsant activity may occur (typically within 3 months of initiation); dose adjustment may be necessary. Periodically reevaluate the long-term usefulness of clonazepam for the individual patient.
- Tolerance: Clonazepam is a long half-life benzodiazepine. Duration of action after a single dose is determined by redistribution rather than metabolism. Tolerance develops to the anticonvulsant effects. It does not develop to the anxiolytic effects (Vinkers 2012). Chronic use of this agent may increase the perioperative benzodiazepine dose needed to achieve desired effect.
- Withdrawal: Rebound or withdrawal symptoms may occur following abrupt discontinuation or large decreases in dose. Use caution when reducing dose or withdrawing therapy; decrease slowly and monitor for withdrawal symptoms. Flumazenil may cause withdrawal in patients receiving long-term benzodiazepine therapy (Brogden 1988).
Monitoring Parameters
CBC, liver and renal function tests (periodically with long-term therapy) suicidality (eg, suicidal thoughts, depression, behavioral changes)
Pregnancy
Pregnancy Considerations
Clonazepam crosses the placenta. Teratogenic effects have been observed with some benzodiazepines; however, additional studies are needed. The incidence of premature birth and low birth weights may be increased following maternal use of benzodiazepines; hypoglycemia and respiratory problems in the neonate may occur following exposure late in pregnancy. Neonatal withdrawal symptoms may occur within days to weeks after birth and “floppy infant syndrome” (which also includes withdrawal symptoms) has been reported with some benzodiazepines, including clonazepam (Bergman 1992; Iqbal 2002; Wikner 2007). A combination of factors influences the potential teratogenicity of anticonvulsant therapy. When treating pregnant females with epilepsy, monotherapy with the lowest effective dose and avoidance medications known to have a high incidence of teratogenic effects is recommended (Harden 2009; Wlodarczyk 2012). When treating pregnant females with panic disorder, psychosocial interventions should be considered prior to pharmacotherapy (APA 2009).
Patients exposed to clonazepam during pregnancy are encouraged to enroll themselves into the AED Pregnancy Registry by calling 1-888-233-2334. Additional information is available at www.aedpregnancyregistry.org.
Patient Education
What is this drug used for?
- It is used to treat seizures.
- It is used to treat panic attacks.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Fatigue
- Increased saliva
- Common cold symptoms
- Constipation
- Dizziness
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Shortness of breath
- Change in balance
- Confusion
- Severe loss of strength and energy
- Trouble with memory
- Seizures
- Menstrual pain
- Nightmares
- Sensing things that seem real but are not
- Depression like thoughts of suicide, anxiety, emotional instability, or confusion.
- Agitation
- Irritability
- Panic attacks
- Behavioral changes
- Mood changes
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.