Boxed Warning
Thromboembolic events:
Thromboembolism, thrombotic and thrombophlebitic events, including sagittal sinus thrombosis and life-threatening or fatal strokes have been reported.
Pregnancy:
Use of danazol in pregnancy is contraindicated. A sensitive test (eg, beta subunit test if available) capable of determining early pregnancy is recommended immediately prior to start of therapy. Additionally, a nonhormonal method of contraception should be used during therapy. If a patient becomes pregnant while taking danazol, administration of the drug should be discontinued and the patient should be apprised of the potential risk to the fetus. Exposure to danazol in utero may result in androgenic effects on the female fetus; reports of clitoral hypertrophy, labial fusion, urogenital sinus defect, vaginal atresia, and ambiguous genitalia have been received.
Hepatic effects:
Experience with long-term therapy with danazol is limited. Peliosis hepatis and benign hepatic adenoma have been observed with long-term use. Peliosis hepatis and hepatic adenoma may be silent until complicated by acute, potentially life-threatening intra-abdominal hemorrhage. The physician therefore should be alert to this possibility. Attempts should be made to determine the lowest dose that will provide adequate protection. If danazol was begun at a time of exacerbation of hereditary angioneurotic edema due to trauma, stress or other cause, periodic attempts to decrease or withdraw therapy should be considered.
Intracranial hypertension:
Danazol has been associated with several cases of benign intracranial hypertension also known as pseudotumor cerebri. Early signs and symptoms of benign intracranial hypertension include papilledema, headache, nausea and vomiting, and visual disturbances. Patients with these symptoms should be screened for papilledema and, if present, the patients should be advised to discontinue danazol immediately and be referred to a neurologist for further diagnosis and care.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Capsule, Oral:
Generic: 50 mg, 100 mg, 200 mg
Pharmacology
Mechanism of Action
Suppresses pituitary output of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), resulting in regression and atrophy of normal and ectopic endometrial tissue; decreases rate of growth of abnormal breast tissue; reduces attacks associated with hereditary angioedema by increasing levels of C4 component of complement
Pharmacokinetics/Pharmacodynamics
Metabolism
Extensively hepatic, primarily to 2-hydroxymethyl danazol and ethisterone
Excretion
Urine and feces
Onset of Action
Immune thrombocytopenia (off-label use): Initial response: 14 to 90 days; Peak response: 28 to 180 days (Neunert 2011)
Time to Peak
Serum: 4 hours (range: 2 to 8 hours)
Half-Life Elimination
9.7 ± 3.29 hours (variable; up to 24 hours following long-term use for endometriosis)
Use: Labeled Indications
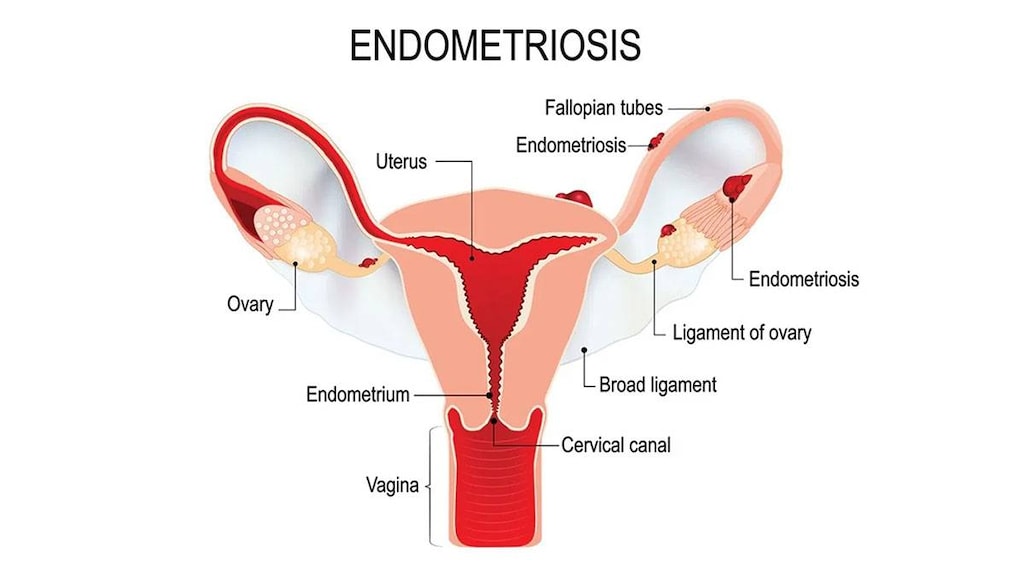
Endometriosis: Treatment of endometriosis amenable to hormonal management.
Hereditary angioedema (HAE), prophylaxis: Prevention of attacks of angioedema of all types (cutaneous, abdominal, laryngeal) in males and females.
Guideline recommendations:Danazol may be considered for short-term pre-procedural and long-term HAE prophylaxis as an alternative to CI inhibitor (human). Danazol is not recommended for treatment of acute HAE attacks (WAO/EAACI [Maurer 2018]).
Use: Off Label
Cyclic breast pain (mastalgia) associated with benign breast disorderscyes
Data from a meta-analysis, which included five randomized controlled studies, support the use of danazol in the treatment of cyclic breast pain (breast pain associated with the menstrual cycle) in females with benign breast disease (malignancy excluded). When compared to placebo, danazol significantly decreased breast pain and improved quality of life; however, significant side effects were observed in the majority of women Groen 2017.
Based on the American College of Obstetricians and Gynecologists guidelines for the Diagnosis and Management of Benign Breast Disorders, danazol is effective for reducing cyclic breast pain. Due to adverse events as well as the limitations of use in females who are or may become pregnant, use is generally reserved for severe or refractory cases ACOG 164 2016.
Immune thrombocytopenia, refractorybyes
In noncontrolled studies and case series/reviews of danazol as secondary treatment in patients with persistent/chronic refractory immune thrombocytopenia (ITP) who failed to respond to corticosteroids, some patients achieved partial or complete response; however, very few patients have shown complete response
Contraindications
Hypersensitivity to danazol or any component of the formulation; undiagnosed abnormal genital bleeding; pregnancy; breastfeeding; porphyria; markedly impaired hepatic, renal, or cardiac function; androgen-dependent tumor; active or history of thrombosis or thromboembolic disease
Canadian labeling: Additional contraindications (not in the US labeling): Genital neoplasia; concomitant administration with simvastatin.
Dosage and Administration
Dosing: Adult
Note: In females, begin treatment during menstruation:
Cyclic breast pain (mastalgia) associated with benign breast disorders (off-label): Oral: 100 mg twice daily starting the first day of the menstrual cycle. Dosing range: 100 to 400 mg/day. Duration of therapy: 3 to 6 months. In some studies, dose was tapered off prior to discontinuation (ACOG 164 2016; Döberl 1984; Hinton 1986; Kontostolis 1997; Mansel 1982; Tobiassen 1984).
Endometriosis (females): Oral:
Mild disease: Initial: 200 to 400 mg/day in 2 divided doses; gradually titrate dosage downward to maintain amenorrhea; continue (uninterrupted) for 3 to 6 months (may extend up to 9 months). If symptoms recur following discontinuation, may reinitiate treatment.
Moderate to severe disease or infertility: Initial: 800 mg/day in 2 divided doses; gradually titrate dosage downward to maintain amenorrhea; continue (uninterrupted) for 3 to 6 months (may extend up to 9 months). If symptoms recur following discontinuation, may reinitiate treatment.
Hereditary angioedema (prophylaxis): Oral: Initial: Note: Use minimum effective dose; frequent short courses may lead to side effects associated with long-term use (WAO/EAACI [Maurer 2018]).
Short-term/preprocedural prophylaxis (off-label dose): 2.5 to 10 mg/kg/day; adjust dose according to patient response (maximum: 600 mg/day) (WAO [Craig 2012]). Administer 5 days before and 2 to 3 days after procedure (WAO/EAACI [Maurer 2018]).
Long-term prophylaxis: 100 mg every other day up to 200 mg 2 to 3 times daily; after favorable initial response, decrease the dosage by 50% or less at intervals of 1 to 3 months or longer if the frequency of attacks dictates. If an attack occurs, increase the dosage by up to 200 mg/day; dosages >200 mg/day for an extended period of time are not recommended due to side effects (WAO [Craig 2012]; WAO/EAACI [Maurer 2018]).
Immune thrombocytopenia, refractory (off-label use): Oral: 200 mg 2 to 4 times/day (10 to 15 mg/kg/day in divided doses) (Provan 2010); initial response is observed at 14 to 90 days; may take up to 6 months for peak response (Neunert 2011; Provan 2010) or 600 mg once daily for at least 6 months followed by 400 mg once daily for 3 months, then (if remission maintained) 200 mg once daily (Maloisel 2004).
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Hereditary angioedema: Limited data available (Zuraw 2013b), not a preferred agent: Adolescents ≥16 years:
Short-term prophylaxis (for patient-specific triggers, medical and dental procedures): Oral: 2.5 to 10 mg/kg/day divided into 3 doses; maximum daily dose: 600 mg/day; begin at least 5 days prior and continue for 2 to 5 days post-procedure (Farkas 2017; JTFPP [Zuraw 2013a]; WAO [Craig 2012])
Long-term prophylaxis: Oral: Initial: 2.5 mg/kg/day; maximum initial daily dose: 50 mg/day; increase slowly every 2 weeks until symptoms controlled, maximum tolerated or maximum recommended dose is reached; maximum daily dose: 5 mg/kg/day up to 200 mg/day. Once control is obtained, reduce interval to every other day or every third day; maximum dose: 100 mg/dose (Farkas 2017; WAO [Craig 2012])
Administration
Endometriosis: Initiate therapy during menstruation or ensure patient is not pregnant while on therapy.
Storage
Store at 20°C to 25°C (68°F to 77°F). Protect from light.
Danazol Images
Drug Interactions
Ajmaline: Androgens may enhance the adverse/toxic effect of Ajmaline. Specifically, the risk for cholestasis may be increased. Monitor therapy
Antidiabetic Agents: Hyperglycemia-Associated Agents may diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
ARIPiprazole: CYP3A4 Inhibitors (Weak) may increase the serum concentration of ARIPiprazole. Management: Monitor for increased aripiprazole pharmacologic effects. Aripiprazole dose adjustments may or may not be required based on concomitant therapy and/or indication. Consult full interaction monograph for specific recommendations. Monitor therapy
C1 inhibitors: Androgens may enhance the thrombogenic effect of C1 inhibitors. Monitor therapy
CarBAMazepine: Danazol may decrease the metabolism of CarBAMazepine. Monitor therapy
Corticosteroids (Systemic): May enhance the fluid-retaining effect of Androgens. Monitor therapy
CycloSPORINE (Systemic): Androgens may enhance the hepatotoxic effect of CycloSPORINE (Systemic). Androgens may increase the serum concentration of CycloSPORINE (Systemic). Consider therapy modification
Dofetilide: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Dofetilide. Monitor therapy
Flibanserin: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Flibanserin. Monitor therapy
HMG-CoA Reductase Inhibitors (Statins): Danazol may increase the serum concentration of HMG-CoA Reductase Inhibitors (Statins). Management: Concurrent use of simvastatin with danazol is contraindicated. Do not exceed 20 mg per day of lovastatin if combined with danazol. Fluvastatin, pravastatin, and rosuvastatin may pose lower risk. Exceptions: Fluvastatin; Pitavastatin; Pravastatin; Rosuvastatin. Consider therapy modification
Lemborexant: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Lemborexant. Management: The maximum recommended dosage of lemborexant is 5 mg, no more than once per night, when coadministered with weak CYP3A4 inhibitors. Consider therapy modification
Lomitapide: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Lomitapide. Management: Patients on lomitapide 5 mg/day may continue that dose. Patients taking lomitapide 10 mg/day or more should decrease the lomitapide dose by half. The lomitapide dose may then be titrated up to a max adult dose of 30 mg/day. Consider therapy modification
NiMODipine: CYP3A4 Inhibitors (Weak) may increase the serum concentration of NiMODipine. Monitor therapy
Pimozide: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Pimozide. Avoid combination
Simvastatin: Danazol may increase the serum concentration of Simvastatin. Avoid combination
Tacrolimus (Systemic): Danazol may increase the serum concentration of Tacrolimus (Systemic). Monitor therapy
Tacrolimus (Topical): Danazol may increase the serum concentration of Tacrolimus (Topical). Monitor therapy
Triazolam: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Triazolam. Management: Consider triazolam dose reduction in patients receiving concomitant weak CYP3A4 inhibitors. Consider therapy modification
Ubrogepant: CYP3A4 Inhibitors (Weak) may increase the serum concentration of Ubrogepant. Management: In patients taking weak CYP3A4 inhibitors, the initial and second dose (if needed) of ubrogepant should be limited to 50 mg. Consider therapy modification
Vitamin D Analogs: Danazol may enhance the hypercalcemic effect of Vitamin D Analogs. Exceptions: Calcipotriene; Calcitriol (Topical); Tacalcitol. Monitor therapy
Vitamin K Antagonists (eg, warfarin): Androgens may enhance the anticoagulant effect of Vitamin K Antagonists. Consider therapy modification
Test Interactions
Estradiol (Kishino 2010); danazol may interfere with laboratory tests for testosterone, androstenedione, and dehydroepiandrosterone
Adverse Reactions
Frequency not defined.
Cardiovascular: Edema, flushing, hypertension, myocardial infarction, palpitations, syncope, tachycardia
Central nervous system: Depression, dizziness, emotional lability, fatigue, headache, nervousness, paresthesia, sleep disorder, voice disorder (deepening of the voice, hoarseness, instability, sore throat)
Dermatologic: Acne vulgaris, alopecia, diaphoresis, maculopapular rash, papular rash, pruritus, seborrhea, urticaria, vesicular eruption
Endocrine & metabolic: Amenorrhea (may continue post-therapy), change in libido, decreased glucose tolerance (and glucagon changes), decreased HDL cholesterol, decreased thyroxine binding globulin, hirsutism (mild), increased LDL cholesterol, increased thyroxine binding globulin, menstrual disease (altered timing of cycle, spotting), weight gain
Gastrointestinal: Constipation, gastroenteritis, nausea, vomiting
Genitourinary: Asthenospermia, breast atrophy, decreased ejaculate volume, hematuria, inhibition of spermatogenesis, spermatozoa disorder (changes in sperm count and semen viscosity), vaginal dryness, vaginal irritation
Hematologic & oncologic: Abnormal erythrocytes (increased), decreased sex hormone binding globulin, eosinophilia, increased sex hormone-binding globulin, leukocytosis, leukopenia, malignant neoplasm (after prolonged use), petechial rash, polycythemia, purpuric rash, thrombocythemia, thrombocytopenia
Hepatic: Cholestatic jaundice, hepatic adenoma, hepatic neoplasm (malignant; after prolonged use), increased liver enzymes, jaundice, peliosis hepatitis
Neuromuscular & skeletal: Ankylosing spondylitis, arthralgia, back pain, increased creatine phosphokinase, joint swelling, limb pain, muscle cramps, muscle spasm, neck pain, tremor, weakness
Ophthalmic: Visual disturbance
Respiratory: Interstitial pneumonitis
<1%, postmarketing, and/or case reports: Anxiety, carpal tunnel syndrome, cataract, change in appetite, chills, clitoromegaly, convulsions, erythema multiforme, fever, gingival hemorrhage, Guillain-Barre syndrome, hepatotoxicity (idiosyncratic) (Chalasani 2014), nasal congestion, nipple discharge, pancreatitis, pelvic pain, pseudotumor cerebri, purpura (splenic peliosis), skin photosensitivity, Stevens-Johnson syndrome
Warnings/Precautions
Concerns related to adverse effects:
- Androgenic effects: May cause nonreversible androgenic effects.
- Blood lipid changes: Anabolic steroids may cause blood lipid changes (decreased high density lipoproteins and increased low density lipoproteins) with increased risk of arteriosclerosis and coronary artery disease.
- Hepatotoxicity: [US Boxed Warning]: Peliosis hepatis and benign hepatic adenoma have been reported with long-term use. Peliosis hepatis and hepatic adenoma may be silent until complicated by acute, potentially life-threatening intraabdominal hemorrhage. Use the lowest effective dose. If therapy was initiated at a time of exacerbation of hereditary angioneurotic edema due to trauma, stress or other cause, consider periodic attempts to decrease or withdraw therapy. Monitor liver function tests and monitor closely for potential hepatotoxicity during therapy. Use is contraindicated in patients with marked hepatic impairment.
- Intracranial hypertension: [US Boxed Warning]: Danazol is associated with cases of benign intracranial hypertension (also known as pseudotumor cerebri). Early signs and symptoms include papilledema, headache, nausea and vomiting, and visual disturbances. Monitor for symptoms; if symptoms occur, screen for papilledema. Discontinue immediately and refer for neurology care if papilledema is present.
- Thromboembolic events: [US Boxed Warning]: Thromboembolism, thrombotic, and thrombophlebitic events have been reported (including sagittal sinus thrombosis and life-threatening or fatal strokes).
Disease-related concerns:
- Cyclic breast pain (mastalgia) associated with benign breast disorders: Use is reserved for severe and refractory cases that have not responded to conservative measures and analgesics. Malignancy should be ruled out prior to therapy (ACOG 164 2016; Groen 2017).
- Diabetes: Use with caution in patients with diabetes mellitus; insulin requirements may be increased; monitor carefully.
- Edematous conditions: Use with caution in patients with conditions influenced by edema (eg, cardiovascular disease, migraine, seizure disorder, renal impairment); danazol may cause fluid retention.
- Porphyria: May cause exacerbations of acute intermittent porphyria; use is contraindicated in patients with porphyria.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pregnancy: [US Boxed Warning]: Danazol use is contraindicated in pregnancy. Pregnancy should be ruled out immediately prior to starting treatment using a sensitive test (eg, beta subunit test if available) capable of determining early pregnancy. A nonhormonal method of contraception should also be used during therapy. If a patient becomes pregnant during danazol treatment, discontinue danazol and apprise the patient of the potential risk to the fetus. Exposure to danazol in utero may result in androgenic effects on the female fetus; reports of clitoral hypertrophy, labial fusion, urogenital sinus defect, vaginal atresia, and ambiguous genitalia have been received.
Other warnings/precautions:
- Appropriate use: Endometriosis: Danazol is generally reserved for the treatment of pain associated with endometriosis when other agents are not available, due to its high incidence of adverse events (Dunselman 2014).
Monitoring Parameters
Liver and renal function tests (periodically); hematologic parameters; lipid panel. Signs and symptoms of intracranial hypertension (papilledema, headache, nausea, vomiting), androgenic changes, and/or fluid retention.
Hereditary angioedema, long-term prophylaxis: CBC, urinalysis, liver function test and lipid profile (baseline, every 6 months while on therapy and 6 months after discontinuation); liver ultrasound (baseline and annually); blood pressure (every 6 months) (WAO [Craig 2012])
Cyclic breast pain (mastalgia) associated with benign breast disorders: Pain and side effects; in most studies this was done (at a minimum) following 1, 3, and 6 months of therapy (Döberl 1984; Hinton 1986; Kontostolis 1997; Mansel 1982; Tobiassen 1984)
Pregnancy
Pregnancy Considerations
Use of danazol in pregnancy is contraindicated. If a patient becomes pregnant while taking danazol, administration of the drug should be discontinued and the patient should be apprised of the potential risk to the fetus. Exposure to danazol in utero may result in androgenic effects on the female fetus; reports of clitoral hypertrophy, labial fusion, urogenital sinus defect, vaginal atresia, and ambiguous genitalia have been received.
The use of danazol for the management of hereditary angioedema (HAE) in pregnancy that is not responsive to preferred therapy has been described in case reports (Altman 2006; Boulos 1994; González-Quevedo 2016; Milingos 2009). However, danazol is contraindicated during pregnancy; current guidelines recommend use of other agents in pregnant females (WAO/EAACI [Maurer 2018]).
[US Boxed Warning]:A sensitive test (eg, beta subunit test, if available) capable of determining early pregnancy is recommended immediately prior to start of therapy. Additionally, a nonhormonal method of contraception should be used during therapy.
Patient Education
What is this drug used for?
- It is used to treat endometriosis.
- It is used to treat fibrocystic breast disease.
- It is used to treat swelling attacks in people with hereditary angioedema (HAE).
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Anxiety
- Flushing
- Sweating a lot
- Hair loss
- Emotional instability
- Acne
- Virilization like in females a deep voice, facial hair, acne, or menstrual changes
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Severe cerebrovascular disease like change in strength on one side is greater than the other, trouble speaking or thinking, change in balance, or vision changes
- Sexual dysfunction
- Vaginal irritation
- Breast size changes
- Swelling
- Weight gain
- Trouble breathing
- Dizziness
- Severe headache
- Nausea
- Vomiting
- Seizures
- Blood clots like numbness or weakness on one side of the body; pain, redness, tenderness, warmth, or swelling in the arms or legs; change in color of an arm or leg; chest pain; shortness of breath; fast heartbeat; or coughing up blood
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.