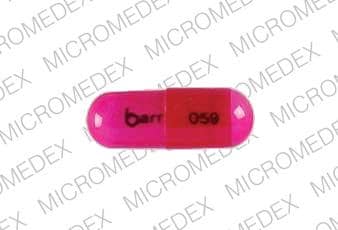
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Capsule, Oral, as hydrochloride:
Allergy Relief: 25 mg [contains brilliant blue fcf (fd&c blue #1), butylparaben, edetate calcium disodium, fd&c blue #2 (indigotine), fd&c red #40, fd&c yellow #10 (quinoline yellow), methylparaben, polysorbate 80, propylparaben]
Banophen: 25 mg, 50 mg [contains brilliant blue fcf (fd&c blue #1), fd&c red #40]
Benadryl Allergy: 25 mg [dye free]
Diphenhist: 25 mg [contains brilliant blue fcf (fd&c blue #1), fd&c red #40]
Genahist: 25 mg
Geri-Dryl: 25 mg
GoodSense Allergy Relief: 25 mg [dye free]
GoodSense Sleep Aid: 50 mg [gluten free; contains brilliant blue fcf (fd&c blue #1)]
Ormir: 50 mg [contains fd&c yellow #10 (quinoline yellow), fd&c yellow #6 (sunset yellow)]
Pharbedryl: 25 mg, 50 mg [contains brilliant blue fcf (fd&c blue #1), fd&c red #40]
Q-Dryl: 25 mg [DSC] [contains brilliant blue fcf (fd&c blue #1), butylparaben, fd&c red #40, methylparaben, propylparaben]
ZzzQuil: 25 mg [contains brilliant blue fcf (fd&c blue #1), fd&c red #40]
Generic: 25 mg, 50 mg
Elixir, Oral, as hydrochloride:
Diphen: 12.5 mg/5 mL (118 mL) [contains alcohol, usp, fd&c red #40; cinnamon-anise flavor]
Generic: 12.5 mg/5 mL (5 mL, 10 mL)
Liquid, Oral, as hydrochloride:
Allergy Childrens: 12.5 mg/5 mL (118 mL, 236 mL, 473 mL) [alcohol free, dye free; contains saccharin sodium, sodium benzoate, sorbitol]
Allergy Relief Childrens: 12.5 mg/5 mL (118 mL, 480 mL) [alcohol free; contains fd&c red #40, sodium benzoate; cherry flavor]
Aurodryl Allergy Childrens: 12.5 mg/5 mL (118 mL) [alcohol free; contains fd&c red #40, sodium benzoate; cherry flavor]
Banophen: 12.5 mg/5 mL (118 mL [DSC]) [alcohol free; cherry flavor]
Banophen: 12.5 mg/5 mL (473 mL [DSC]) [alcohol free, sugar free; cherry flavor]
Benadryl Allergy Childrens: 12.5 mg/5 mL (118 mL, 236 mL) [alcohol free; contains fd&c red #40, sodium benzoate]
Benadryl Allergy Childrens: 12.5 mg/5 mL (236 mL) [alcohol free; contains fd&c red #40, sodium benzoate; cherry flavor]
Benadryl Allergy Childrens: 12.5 mg/5 mL (118 mL) [alcohol free, dye free, sugar free; contains saccharin sodium, sodium benzoate]
Diphenhist: 12.5 mg/5 mL (118 mL [DSC], 473 mL [DSC]) [alcohol free; contains fd&c red #40, saccharin sodium, sodium benzoate; fruit flavor]
Geri-Dryl: 12.5 mg/5 mL (473 mL) [alcohol free; contains fd&c red #40, methylparaben, propylene glycol, propylparaben]
Geri-Dryl: 12.5 mg/5 mL (473 mL) [alcohol free; contains fd&c red #40, saccharin sodium, sodium benzoate]
M-Dryl: 12.5 mg/5 mL (120 mL, 473 mL) [alcohol free; contains fd&c red #40, propylene glycol, saccharin sodium, sodium benzoate; cherry flavor]
Naramin: 12.5 mg/5 mL (5 mL) [alcohol free; contains fd&c red #40, sodium benzoate; cherry flavor]
PediaCare Childrens Allergy: 12.5 mg/5 mL (118 mL) [alcohol free; contains fd&c red #40, sodium benzoate]
Q-Dryl: 12.5 mg/5 mL (118 mL [DSC], 237 mL [DSC], 473 mL [DSC]) [alcohol free; contains fd&c red #40, saccharin sodium, sodium benzoate; cherry flavor]
Scot-Tussin Allergy Relief: 12.5 mg/5 mL (118.3 mL [DSC], 240 mL [DSC], 480 mL [DSC], 3780 mL [DSC]) [alcohol free, dye free, saccharin free, sodium free, sorbitol free, sugar free]
Siladryl Allergy: 12.5 mg/5 mL (118 mL, 237 mL, 473 mL) [alcohol free, sugar free; contains fd&c red #40, methylparaben, propylene glycol, propylparaben, saccharin sodium; cherry flavor]
Total Allergy Medicine: 12.5 mg/5 mL (118 mL) [alcohol free]
ZzzQuil: 50 mg/30 mL (177 mL, 354 mL) [contains alcohol, usp, brilliant blue fcf (fd&c blue #1), fd&c red #40, propylene glycol, saccharin sodium, sodium benzoate; berry flavor]
ZzzQuil: 50 mg/30 mL (354 mL) [contains alcohol, usp, brilliant blue fcf (fd&c blue #1), fd&c red #40, propylene glycol, saccharin sodium, sodium benzoate; vanilla cherry flavor]
ZzzQuil: 50 mg/30 mL (177 mL, 354 mL) [alcohol free; contains brilliant blue fcf (fd&c blue #1), propylene glycol, saccharin sodium, sodium benzoate; mango berry flavor]
Generic: 12.5 mg/5 mL (5 mL, 10 mL, 118 mL, 473 mL); 6.25 mg/mL (30 mL)
Solution, Injection, as hydrochloride:
Generic: 50 mg/mL (1 mL, 10 mL)
Solution, Injection, as hydrochloride [preservative free]:
Generic: 50 mg/mL (1 mL)
Strip, Oral, as hydrochloride:
Triaminic Cough/Runny Nose: 12.5 mg (16 ea [DSC]) [contains alcohol, usp, brilliant blue fcf (fd&c blue #1), fd&c red #40; grape flavor]
Syrup, Oral, as hydrochloride:
Quenalin: 12.5 mg/5 mL (120 mL [DSC])
Silphen Cough: 12.5 mg/5 mL (118 mL [DSC], 237 mL [DSC], 473 mL [DSC]) [contains alcohol, usp, fd&c red #40, menthol, methylparaben, propylene glycol, propylparaben; strawberry flavor]
Tablet, Oral, as hydrochloride:
Aler-Dryl: 50 mg
Allergy Relief: 25 mg [contains polysorbate 80]
Anti-Hist Allergy: 25 mg
Banophen: 25 mg
Benadryl Allergy: 25 mg
CareAll Comp Allergy Relief: 25 mg
Complete Allergy Medication: 25 mg [DSC]
Complete Allergy Relief: 25 mg [DSC]
Diphen: 25 mg
Diphenhist: 25 mg [DSC]
Geri-Dryl: 25 mg
Nighttime Sleep Aid: 25 mg
Nytol: 25 mg
Nytol Maximum Strength: 50 mg
Simply Sleep: 25 mg [contains brilliant blue fcf (fd&c blue #1)]
Sleep Tabs: 25 mg [scored; contains fd&c blue #1 aluminum lake]
Sominex: 25 mg [DSC] [contains fd&c blue #1 aluminum lake]
Tetra-Formula Nighttime Sleep: 50 mg [contains fd&c blue #1 aluminum lake]
Total Allergy: 25 mg
Generic: 25 mg
Tablet Chewable, Oral, as hydrochloride:
Benadryl Allergy Childrens: 12.5 mg [contains fd&c blue #1 aluminum lake]
Pharmacology
Mechanism of Action
Competes with histamine for H1-receptor sites on effector cells in the gastrointestinal tract, blood vessels, and respiratory tract; anticholinergic and sedative effects are also seen
Pharmacokinetics/Pharmacodynamics
Distribution
Vd: Children: 22 L/kg (range: 15 to 28 L/kg); Adults: 17 L/kg (range: 13 to 20 L/kg); Elderly: 14 L/kg (range: 7 to 20 L/kg) (Blyden 1986; Simons 1990)
Metabolism
Extensively hepatic n-demethylation via CYP2D6; minor demethylation via CYP1A2, 2C9 and 2C19; smaller degrees in pulmonary and renal systems; significant first-pass effect (Akutsu 2007)
Excretion
Urine (as metabolites and unchanged drug) (Albert 1975; Maurer 1988)
Time to Peak
Serum: ~2 hours (Blyden 1986; Simons 1990)
Duration of Action
Histamine-induced wheal suppression: ≤10 hours (Simons 1990)
Histamine-induced flare suppression: ≤12 hours (Simons 1990)
Half-Life Elimination
Children: 5 hours (range: 4 to 7 hours); Adults: 9 hours (range: 7 to 12 hours); Elderly: 13.5 hours (range: 9 to 18 hours) (Blyden 1986; Simons 1990)
Protein Binding
98.5% (Vozeh 1988)
Use: Labeled Indications
Symptomatic relief of allergic symptoms caused by histamine release, including nasal allergies and allergic dermatosis; adjunct to epinephrine in the treatment of anaphylaxis; insomnia, occasional; prevention or treatment of motion sickness; antitussive; management of parkinsonian syndrome including drug-induced extrapyramidal symptoms (dystonic reactions) alone or in combination with centrally acting anticholinergic agents
Guideline recommendations:
Anaphylaxis: Antihistamines are considered second-line treatment only after epinephrine administration in the adjunct management of anaphylaxis (AAAAI [Lieberman 2015]).
Insomnia: American Academy of Sleep Medicine guidelines for the treatment of chronic insomnia suggest diphenhydramine not be used for sleep-onset or sleep-maintenance insomnia in adults due to the absence of evidence for clinically significant improvement (AASM [Sateia 2017]).
Use: Off Label
Angioedema, allergic (acute)c
Clinical experience suggests the utility of diphenhydramine in the management of allergic angioedema James 2017, Zuraw 2019. Diphenhydramine should not be used as monotherapy for angioedema with anaphylaxis; use epinephrine if anaphylaxis symptoms are present.
Infusion- or transfusion-related reactionsc
Clinical experience suggests the utility of diphenhydramine for the prevention of infusion-related reactions and in the treatment of infusion- or transfusion-related reactions as an adjunct to other appropriate measures (eg, stopping the infusion, reducing the infusion rate). An optimal premedication regimen has not been identified; refer to institutional protocols Castells 2019. Diphenhydramine should not be used as initial or sole treatment of anaphylaxis; use epinephrine if anaphylaxis symptoms are present.
Nausea and vomiting, pregnancy associated, severe or refractorybyes
Based on the Society of Obstetricians and Gynaecologists of Canada guideline on nausea and vomiting of pregnancy and the American College of Obstetricians and Gynecologists practice bulletin, use of diphenhydramine may be considered in the treatment of refractory nausea and vomiting when preferred agents do not provide initial symptom improvement ACOG 2018, Arsenault 2002, Magee 2002.
Scombroid (histamine) poisoningc
Clinical experience suggests the utility of diphenhydramine in the treatment of scombroid (histamine) poisoning Taylor 1989.
Urticariayes
Based on the European Academy of Allergology and Clinical Immunology/Global Asthma and Allergy European Network/European Dermatology Forum/World Allergy Organization guidelines for the management of urticaria, diphenhydramine may be considered in the treatment of acute and chronic urticaria Zuberbier 2018.
Vertigo, acute treatmentc
Clinical experience suggests the utility of diphenhydramine in the acute treatment of vertigo Furman 2019, Zajonc 2006.
Contraindications
Hypersensitivity to diphenhydramine, other structurally related antihistamines, or any component of the formulation; neonates or premature infants; breast-feeding
Additional contraindications: Parenteral: Use as a local anesthetic
OTC labeling: When used for self-medication, do not use in children <6 years, to make a child sleep, or with any other diphenhydramine-containing products (including topical products)
Dosage and Administration
Dosing: Adult
Note: Dosing recommendations based on clinical experience and general dosing range in manufacturer's labeling unless otherwise noted. Although oral dosing up to 50 mg every 4 hours (maximum: 300 mg/day) and parenteral dosing up to 100 mg every 6 hours (maximum: 400 mg/day) are approved in some labels, adverse effects may be increased at these higher doses. In general, clinical experience dictates that the doses provided below are sufficient.
Allergic reactions:
Anaphylaxis (adjunct to epinephrine): Note: Administer epinephrine first when treating anaphylaxis. Do not use diphenhydramine for initial or sole treatment of anaphylaxis because H1 antihistamines do not relieve upper or lower airway obstruction, hypotension, or shock.
IV: 25 to 50 mg given after epinephrine (AAAAI [Lieberman 2015]; Simons 2011).
Mild to moderate cutaneous reactions (eg, pruritus): Note: May combine with a topical or systemic glucocorticoid and/or H2 antihistamine, depending on the severity and/or cause of the allergic reaction.
Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed.
IM, IV: 10 to 50 mg per dose every 6 hours as needed.
Angioedema, allergic (acute) (alternative agent) (off-label use): Note: Should not be used as monotherapy for angioedema with anaphylaxis; use epinephrine, prior to diphenhydramine, if anaphylaxis symptoms are present (James 2017; Zuraw 2019).
Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed.
IM, IV: 10 to 50 mg per dose every 6 hours as needed.
Common cold symptoms (eg, rhinitis, rhinorrhea, sneezing) or upper airway cough syndrome: Note: Used in combination with a decongestant; adverse effects of monotherapy likely outweigh benefits (Björnsdóttir 2007; De Sutter 2012; De Sutter 2015; Irwin 2006; Weinberger 2019).
Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed (in combination with a decongestant); doses at the higher end of the dosing range may not be tolerated.
Dystonic reactions, medication-induced:
Acute treatment: Note: Parenteral administration is preferred for initial treatment of acute symptoms. Treatment should continue with oral administration for several days to prevent recurrence. Duration of anticholinergic therapy should be based on the severity of the dystonic reaction and pharmacokinetic properties (eg, half-life) of the causative agent (Holloman 1997; Lavonas 2019; Marder 2019; Tonda 1994).
Initial dose: IM, IV: 25 to 50 mg once (Lavonas 2019).
Subsequent doses: Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours, typically for 2 to 3 days (Lavonas 2019).
Prophylaxis: Note: May be useful for preventing acute dystonic reactions when administering drugs with increased risk of causing such reactions (eg, haloperidol, metoclopramide) (Kreyenbuhl 2009).
IM, IV: 12.5 to 25 mg prior to administration of high-risk medication (Friedman 2009; Kelley 2012; Kreyenbuhl 2009; Marder 2019).
Infusion- or transfusion-related reactions (off-label use):
Premedication: Note: Typically administered prior to the infusion of certain chemotherapy agents, biologics, or other drugs (eg, contrast media); may be given with a glucocorticoid, acetaminophen, and/or an H2 antihistamine. An optimal premedication regimen has not been identified; refer to institutional protocols (Castells 2019). The routine use of premedication with diphenhydramine prior to blood transfusions to prevent febrile nonhemolytic or allergic transfusion reactions is not recommended or supported by data (Silvergleid 2019; Tobian 2007).
IV, Oral: 25 to 50 mg given ~30 to 60 minutes prior to infusion; use of the oral route may require earlier administration (eg, ~1 to 2 hours prior to infusion) to allow for adequate concentrations at the time of infusion or potential reaction.
Treatment: Note: Administer epinephrine first when treating anaphylaxis. Do not use diphenhydramine for initial or sole treatment of anaphylaxis because H1 antihistamines do not relieve upper or lower airway obstruction, hypotension, or shock. Diphenhydramine should be used for symptomatic relief as an adjunct to other appropriate measures (eg, stopping the infusion, reducing the infusion rate).
IV, Oral: 25 to 50 mg; after 15 to 30 minutes, if symptoms persist, may repeat dose as needed. Do not exceed 100 mg within a 1-hour period (Silvergleid 2019). Note: With oral administration, onset of action is delayed.
Insomnia, occasional (alternative agent): Note: Routine use is not recommended (AASM [Sateia 2017]).
Oral: 25 to 50 mg at bedtime for occasional use only.
Motion sickness:
Prophylaxis: Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed. Note: Antihistamines may be administered 30 to 60 minutes before motion (Dramamine labeling 2019).
Treatment:
Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed.
IM, IV: 10 to 50 mg per dose every 6 hours as needed.
Nausea and vomiting, pregnancy associated, severe or refractory (alternative agent) (off-label use): Note: May be considered as a component of combination therapy when symptoms persist following initial pharmacologic therapy (ACOG 2018).
Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed (ACOG 2018; Magee 2002).
IV: 10 to 50 mg every 6 hours as needed; some experts administer as often as every 4 hours as needed (Smith 2019).
Scombroid (histamine) poisoning (off-label use): Note: For patients with uncomfortable symptoms (eg, flushing, burning, rash) but without respiratory distress or hypotension (Taylor 1989).
Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed.
IM, IV: 10 to 50 mg per dose every 6 hours as needed.
Urticaria (alternative agent): Note: Second-generation antihistamines are preferred (Zuberbier 2018); may consider use in young, healthy patients (eg, administered at bedtime with daytime use of a second-generation antihistamine) (Asero 2019; Khan 2019).
Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed. Alternatively, may administer 25 to 50 mg once daily at bedtime with daytime use of a second-generation antihistamine (Khan 2019).
IM, IV: 10 to 50 mg per dose every 6 hours as needed; some experts administer as often as every 4 hours as needed (Asero 2019).
Vertigo, acute treatment (off-label use):
Oral: 25 mg every 4 to 6 hours or 50 mg every 6 to 8 hours as needed (Furman 2019; Zajonc 2006).
IM, IV: 10 to 50 mg per dose every 6 hours as needed (Furman 2019; Zajonc 2006).
Duration: Discontinue as soon as severe symptoms resolve, usually after 1 to 2 days (Furman 2019).
Dosing: Pediatric
Note: Oral solutions are available in 2 concentrations (ie, 12.5 mg/5 mL and 50 mg/30 mL [eg, ZzzQuil]); precautions should be taken to verify and avoid confusion between the different concentrations; dose should be clearly presented as "mg;" the 50 mg/30 mL oral solution is only indicated for the occasional treatment of insomnia. Although 300 mg/day is listed in prescribing information, some experts suggest that 200 mg/day is adequate for most indications.
Allergic reaction (severe)/anaphylaxis (adjunct to epinephrine): Note: Do not use as initial therapy; antihistamines are considered second- or third-line agents in the treatment of anaphylaxis (AAAAI/ACAAI [Lieberman 2015]; WAO [Simons 2011]; WAO [Simons 2015]).
Infants, Children, and Adolescents: IV, IM, Oral: 1 to 2 mg/kg/dose; may repeat every 6 hours; maximum dose: 50 mg/dose (AAAAI/ACAAI [Lieberman 2015]; Hegenbarth 2008; Liberman 2008; WHO [Simons 2011]).
Allergies; antihistamine; hay fever:
Weight-based dosing: Infants, Children, and Adolescents: Oral: 5 mg/kg/day divided into 3 to 4 doses; maximum daily dose: 300 mg/day; age-related maximum daily doses may also be considered: <6 years: 37.5 mg/day (Kliegman 2011); 6 to 11 years: 150 mg/day; ≥12 years: 300 mg/day.
Fixed dosing: Oral:
Children 2 to <6 years: Limited data available: 6.25 mg every 4 to 6 hours (Kliegman 2011).
Children 6 to <12 years: 12.5 to 25 mg every 4 to 6 hours.
Children ≥12 years and Adolescents: 25 to 50 mg every 4 to 6 hours.
Dystonic reactions/parkinsonism: Infants, Children, and Adolescents:
Initial: IV, IM: 1 to 2 mg/kg/dose; maximum dose: 50 mg/dose; may repeat if necessary (Fuhrman 2015; Hegenbarth 2008).
Subsequent doses:
IV: 5 mg/kg/day in divided doses every 6 hours; Note: Usual adult dose: 25 to 50 mg/dose.
Oral: 5 mg/kg/day divided into 3 to 4 doses; Note: Usual adult dose: 25 to 50 mg/dose.
Insomnia; occasional: Note: Evaluation of sleep hygiene and use of non-pharmacologic (ie, behavioral) treatment are preferred treatments; medications may be used as adjunct treatment (Owens 2005).
Children ≥2 years and Adolescents: Limited data available for <12 years: Oral: 0.5 to 1 mg/kg administered 30 minutes before bedtime; usual dose: 12.5 to 50 mg/dose; maximum dose: 50 mg/dose (Felt 2014; Russo 1976).
Motion sickness:
Prophylaxis: Note: First dose should be administered 30 to 60 minutes before travel (CDC 2017).
Weight-based dosing:
Infants and Children <2 years: Oral: 5 mg/kg/day divided into 3 to 4 doses; infants and toddlers are generally immune to motion sickness (CDC 2017).
Children ≥2 years: Oral: 0.5 to 1 mg/kg/dose every 6 hours; maximum dose: 25 mg/dose (CDC 2017).
Fixed dosing: Children and Adolescents: Oral: 12.5 to 25 mg 3 to 4 times daily.
Treatment: Infants, Children, and Adolescents:
IV, IM: 1.25 mg/kg/dose every 6 hours. Note: Usual adult dose: 10 to 50 mg/dose.
Oral: 5 mg/kg/day divided into 3 to 4 doses; usual dose: 12.5 to 25 mg/dose.
Urticaria/pruritus: Infants, Children, and Adolescents: Oral: 5 mg/kg/day divided into 3 to 4 doses; usual dose: 12.5 to 25 mg/dose; maximum daily dose: 300 mg/day; Note: Guidelines do not recommend as first-line treatment for chronic urticaria (EAACI/GA2LEN/EDF/WAO [Zuberbier 2018]).
Administration
Oral: When used to prevent motion sickness, first dose should be given 30 minutes prior to exposure. When used for occasional insomnia, dose should be given 30 minutes before bedtime.
IM, IV: Injection solution is for IV or deep IM administration only. For IV administration, inject at a rate ≤25 mg/minute. Local necrosis may result with SubQ or intradermal use.
Dietary Considerations
Some products may contain sodium and/or phenylalanine.
Storage
Injection: Store at room temperature of 20°C to 25°C (68°F to 77°F); protect from light and freezing.
Oral: Store at room temperature. Protect capsules and tablets from moisture. Protect oral solution from freezing and light.
DiphenhydrAMINE (Systemic) Images
Drug Interactions
Acetylcholinesterase Inhibitors: May diminish the therapeutic effect of Anticholinergic Agents. Anticholinergic Agents may diminish the therapeutic effect of Acetylcholinesterase Inhibitors. Monitor therapy
Aclidinium: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Alcohol (Ethyl): CNS Depressants may enhance the CNS depressant effect of Alcohol (Ethyl). Monitor therapy
Alizapride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Amantadine: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Amezinium: Antihistamines may enhance the stimulatory effect of Amezinium. Monitor therapy
Amphetamines: May diminish the sedative effect of Antihistamines. Monitor therapy
Anticholinergic Agents: May enhance the adverse/toxic effect of other Anticholinergic Agents. Monitor therapy
ARIPiprazole: CYP2D6 Inhibitors (Weak) may increase the serum concentration of ARIPiprazole. Management: Monitor for increased aripiprazole pharmacologic effects. Aripiprazole dose adjustments may or may not be required based on concomitant therapy and/or indication. Consult full interaction monograph for specific recommendations. Monitor therapy
Azelastine (Nasal): CNS Depressants may enhance the CNS depressant effect of Azelastine (Nasal). Avoid combination
Benzylpenicilloyl Polylysine: Antihistamines may diminish the diagnostic effect of Benzylpenicilloyl Polylysine. Management: Suspend systemic H1 antagonists for benzylpenicilloyl-polylysine skin testing and delay testing until systemic antihistaminic effects have dissipated. A histamine skin test may be used to assess persistent antihistaminic effects. Consider therapy modification
Betahistine: Antihistamines may diminish the therapeutic effect of Betahistine. Monitor therapy
Blonanserin: CNS Depressants may enhance the CNS depressant effect of Blonanserin. Consider therapy modification
Botulinum Toxin-Containing Products: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Brexanolone: CNS Depressants may enhance the CNS depressant effect of Brexanolone. Monitor therapy
Brimonidine (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Bromopride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Bromperidol: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
Buprenorphine: CNS Depressants may enhance the CNS depressant effect of Buprenorphine. Management: Consider reduced doses of other CNS depressants, and avoiding such drugs in patients at high risk of buprenorphine overuse/self-injection. Initiate buprenorphine at lower doses in patients already receiving CNS depressants. Consider therapy modification
Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Cannabis: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Chloral Betaine: May enhance the adverse/toxic effect of Anticholinergic Agents. Monitor therapy
Chlormethiazole: May enhance the CNS depressant effect of CNS Depressants. Management: Monitor closely for evidence of excessive CNS depression. The chlormethiazole labeling states that an appropriately reduced dose should be used if such a combination must be used. Consider therapy modification
Chlorphenesin Carbamate: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
Cimetropium: Anticholinergic Agents may enhance the anticholinergic effect of Cimetropium. Avoid combination
CNS Depressants: May enhance the adverse/toxic effect of other CNS Depressants. Monitor therapy
Dimethindene (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Doxylamine: May enhance the CNS depressant effect of CNS Depressants. Management: The manufacturer of Diclegis (doxylamine/pyridoxine), intended for use in pregnancy, specifically states that use with other CNS depressants is not recommended. Monitor therapy
Dronabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Droperidol: May enhance the CNS depressant effect of CNS Depressants. Management: Consider dose reductions of droperidol or of other CNS agents (eg, opioids, barbiturates) with concomitant use. Exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Consider therapy modification
Eluxadoline: Anticholinergic Agents may enhance the constipating effect of Eluxadoline. Avoid combination
Esketamine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Flunitrazepam: CNS Depressants may enhance the CNS depressant effect of Flunitrazepam. Consider therapy modification
Gastrointestinal Agents (Prokinetic): Anticholinergic Agents may diminish the therapeutic effect of Gastrointestinal Agents (Prokinetic). Monitor therapy
Glucagon: Anticholinergic Agents may enhance the adverse/toxic effect of Glucagon. Specifically, the risk of gastrointestinal adverse effects may be increased. Monitor therapy
Glycopyrrolate (Oral Inhalation): Anticholinergic Agents may enhance the anticholinergic effect of Glycopyrrolate (Oral Inhalation). Avoid combination
Glycopyrronium (Topical): May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Hyaluronidase: Antihistamines may diminish the therapeutic effect of Hyaluronidase. Management: Patients receiving antihistamines (particularly at larger doses) may not experience the desired clinical response to standard doses of hyaluronidase. Larger doses of hyaluronidase may be required. Consider therapy modification
HYDROcodone: CNS Depressants may enhance the CNS depressant effect of HYDROcodone. Management: Avoid concomitant use of hydrocodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
HydrOXYzine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Ipratropium (Oral Inhalation): May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Itopride: Anticholinergic Agents may diminish the therapeutic effect of Itopride. Monitor therapy
Kava Kava: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
Lemborexant: May enhance the CNS depressant effect of CNS Depressants. Management: Dosage adjustments of lemborexant and of concomitant CNS depressants may be necessary when administered together because of potentially additive CNS depressant effects. Close monitoring for CNS depressant effects is necessary. Consider therapy modification
Levosulpiride: Anticholinergic Agents may diminish the therapeutic effect of Levosulpiride. Avoid combination
Lofexidine: May enhance the CNS depressant effect of CNS Depressants. Management: Drugs listed as exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Monitor therapy
Magnesium Sulfate: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Methotrimeprazine: CNS Depressants may enhance the CNS depressant effect of Methotrimeprazine. Methotrimeprazine may enhance the CNS depressant effect of CNS Depressants. Management: Reduce adult dose of CNS depressant agents by 50% with initiation of concomitant methotrimeprazine therapy. Further CNS depressant dosage adjustments should be initiated only after clinically effective methotrimeprazine dose is established. Consider therapy modification
MetyroSINE: CNS Depressants may enhance the sedative effect of MetyroSINE. Monitor therapy
Mianserin: May enhance the anticholinergic effect of Anticholinergic Agents. Monitor therapy
Minocycline (Systemic): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Mirabegron: Anticholinergic Agents may enhance the adverse/toxic effect of Mirabegron. Monitor therapy
Nabilone: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Nitroglycerin: Anticholinergic Agents may decrease the absorption of Nitroglycerin. Specifically, anticholinergic agents may decrease the dissolution of sublingual nitroglycerin tablets, possibly impairing or slowing nitroglycerin absorption. Monitor therapy
Opioid Agonists: CNS Depressants may enhance the CNS depressant effect of Opioid Agonists. Management: Avoid concomitant use of opioid agonists and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Orphenadrine: CNS Depressants may enhance the CNS depressant effect of Orphenadrine. Avoid combination
Oxatomide: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Oxomemazine: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
OxyCODONE: CNS Depressants may enhance the CNS depressant effect of OxyCODONE. Management: Avoid concomitant use of oxycodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Paraldehyde: CNS Depressants may enhance the CNS depressant effect of Paraldehyde. Avoid combination
Perampanel: May enhance the CNS depressant effect of CNS Depressants. Management: Patients taking perampanel with any other drug that has CNS depressant activities should avoid complex and high-risk activities, particularly those such as driving that require alertness and coordination, until they have experience using the combination. Consider therapy modification
Perhexiline: CYP2D6 Inhibitors (Weak) may increase the serum concentration of Perhexiline. Monitor therapy
Piribedil: CNS Depressants may enhance the CNS depressant effect of Piribedil. Monitor therapy
Pitolisant: Antihistamines may diminish the therapeutic effect of Pitolisant. Avoid combination
Potassium Chloride: Anticholinergic Agents may enhance the ulcerogenic effect of Potassium Chloride. Management: Patients on drugs with substantial anticholinergic effects should avoid using any solid oral dosage form of potassium chloride. Avoid combination
Potassium Citrate: Anticholinergic Agents may enhance the ulcerogenic effect of Potassium Citrate. Avoid combination
Pramipexole: CNS Depressants may enhance the sedative effect of Pramipexole. Monitor therapy
Pramlintide: May enhance the anticholinergic effect of Anticholinergic Agents. These effects are specific to the GI tract. Consider therapy modification
Ramosetron: Anticholinergic Agents may enhance the constipating effect of Ramosetron. Monitor therapy
Revefenacin: Anticholinergic Agents may enhance the anticholinergic effect of Revefenacin. Avoid combination
ROPINIRole: CNS Depressants may enhance the sedative effect of ROPINIRole. Monitor therapy
Rotigotine: CNS Depressants may enhance the sedative effect of Rotigotine. Monitor therapy
Rufinamide: May enhance the adverse/toxic effect of CNS Depressants. Specifically, sleepiness and dizziness may be enhanced. Monitor therapy
Secretin: Anticholinergic Agents may diminish the therapeutic effect of Secretin. Management: Avoid concomitant use of anticholinergic agents and secretin. Discontinue anticholinergic agents at least 5 half-lives prior to administration of secretin. Consider therapy modification
Selective Serotonin Reuptake Inhibitors: CNS Depressants may enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Specifically, the risk of psychomotor impairment may be enhanced. Monitor therapy
Sodium Oxybate: May enhance the CNS depressant effect of CNS Depressants. Management: Consider alternatives to combined use. When combined use is needed, consider minimizing doses of one or more drugs. Use of sodium oxybate with alcohol or sedative hypnotics is contraindicated. Consider therapy modification
Suvorexant: CNS Depressants may enhance the CNS depressant effect of Suvorexant. Management: Dose reduction of suvorexant and/or any other CNS depressant may be necessary. Use of suvorexant with alcohol is not recommended, and the use of suvorexant with any other drug to treat insomnia is not recommended. Consider therapy modification
Tapentadol: May enhance the CNS depressant effect of CNS Depressants. Management: Avoid concomitant use of tapentadol and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Tetrahydrocannabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Tetrahydrocannabinol and Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Thalidomide: CNS Depressants may enhance the CNS depressant effect of Thalidomide. Avoid combination
Thiazide and Thiazide-Like Diuretics: Anticholinergic Agents may increase the serum concentration of Thiazide and Thiazide-Like Diuretics. Monitor therapy
Tiotropium: Anticholinergic Agents may enhance the anticholinergic effect of Tiotropium. Avoid combination
Topiramate: Anticholinergic Agents may enhance the adverse/toxic effect of Topiramate. Monitor therapy
Trimeprazine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Umeclidinium: May enhance the anticholinergic effect of Anticholinergic Agents. Avoid combination
Zolpidem: CNS Depressants may enhance the CNS depressant effect of Zolpidem. Management: Reduce the Intermezzo brand sublingual zolpidem adult dose to 1.75 mg for men who are also receiving other CNS depressants. No such dose change is recommended for women. Avoid use with other CNS depressants at bedtime; avoid use with alcohol. Consider therapy modification
Test Interactions
May interfere with urine detection of methadone and phencyclidine (false-positives); may cause false-positive serum TCA screen; may suppress the wheal and flare reactions to skin test antigens
Adverse Reactions
Frequency not defined:
Cardiovascular: Chest tightness, extrasystoles, hypotension, palpitations, tachycardia
Central nervous system: Ataxia, chills, confusion, dizziness, drowsiness, euphoria, excitement, fatigue, headache, irritability, nervousness, neuritis, paradoxical excitation, paresthesia, restlessness, sedation, seizure, vertigo
Dermatologic: Diaphoresis, skin photosensitivity, skin rash, urticaria
Gastrointestinal: Anorexia, constipation, diarrhea, dry mucous membranes, epigastric distress, nausea, vomiting, xerostomia
Genitourinary: Difficulty in micturition, urinary frequency, urinary retention
Hematologic & oncologic: Agranulocytosis, hemolytic anemia, thrombocytopenia
Hypersensitivity: Anaphylactic shock
Neuromuscular & skeletal: Tremor
Ophthalmic: Blurred vision, diplopia
Respiratory: Nasal congestion, pharyngeal edema, thickening of bronchial secretions, wheezing
Warnings/Precautions
Concerns related to adverse effects:
- CNS depression: May cause CNS depression, which may impair physical or mental abilities; patients must be cautioned about performing tasks which require mental alertness (eg, operating machinery or driving).
Disease-related concerns:
- Asthma: Use with caution in patients with a history of asthma.
- Cardiovascular disease: Use with caution in patients with cardiovascular disease (including hypertension and ischemic heart disease).
- Increased intraocular pressure/glaucoma: Use with caution in patients with increased intraocular pressure or angle-closure glaucoma.
- Prostatic hyperplasia/urinary obstruction: Use with caution in patients with prostatic hyperplasia, bladder neck obstruction, and/or GU obstruction.
- Pyloroduodenal obstruction: Use with caution in patients with pyloroduodenal obstruction (including stenotic peptic ulcer).
- Thyroid dysfunction: Use with caution in patients with thyroid dysfunction.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pediatric: Antihistamines may cause excitation in young children. Toxicity (overdose) in pediatric patients may result in hallucinations, convulsions, or death; neonates and young children are highly sensitive to depressive effects of diphenhydramine; use is contraindicated in neonates and premature infants.
Dosage form specific issues:
- Alcohol: Some oral liquid products may contain alcohol.
- Benzyl alcohol and derivatives: Some dosage forms may contain sodium benzoate/benzoic acid; benzoic acid (benzoate) is a metabolite of benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP ["Inactive" 1997]; CDC, 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors, 2001); avoid or use dosage forms containing benzyl alcohol derivative with caution in neonates. See manufacturer’s labeling.
- Oral liquid: Oral solutions are available in two concentrations (ie, 12.5 mg/5 mL and 50 mg/30 mL [eg, ZzzQuil]); precautions should be taken to verify and avoid confusion between the different concentrations; dose should be clearly presented as "mg"; the 50 mg/30 mL oral solution is indicated for the occasional treatment of insomnia.
- Parenteral: Subcutaneous or intradermal use has been associated with tissue necrosis; administer IV or IM only.
- Phenylalanine: Some products may contain phenylalanine.
- Polysorbate 80: Some dosage forms may contain polysorbate 80 (also known as Tweens). Hypersensitivity reactions, usually a delayed reaction, have been reported following exposure to pharmaceutical products containing polysorbate 80 in certain individuals (Isaksson, 2002; Lucente 2000; Shelley, 1995). Thrombocytopenia, ascites, pulmonary deterioration, and renal and hepatic failure have been reported in premature neonates after receiving parenteral products containing polysorbate 80 (Alade, 1986; CDC, 1984). See manufacturer’s labeling.
- Propylene glycol: Some dosage forms may contain propylene glycol; large amounts are potentially toxic and have been associated hyperosmolality, lactic acidosis, seizures, and respiratory depression; use caution (AAP ["Inactive" 1997]; Zar, 2007).
- Soy protein: Some preparations contain soy protein; avoid use in patients with soy protein or peanut allergies.
Other warnings/precautions:
- Self-medication (OTC use): Do not use with other products containing diphenhydramine, even ones used on the skin. Oral products are not for OTC use in children <6 years of age.
Monitoring Parameters
Relief of symptoms, mental alertness
Pregnancy
Pregnancy Risk Factor
B
Pregnancy Considerations
Diphenhydramine crosses the placenta (Miller 2000; Parkin 1974).
In general, the use of first generation antihistamines immediately before parturition may cause respiratory depression in the newborn (Zuberbier 2014).
Diphenhydramine may be used for the treatment of allergic conditions in pregnant women when a first generation antihistamine is indicated (Babalola 2013; Murase 2014; Zuberbier 2014). Diphenhydramine may be used as adjunctive therapy in the management of nausea and vomiting of pregnancy when the preferred agents do not provide initial symptom improvement (ACOG 189 2018). Antihistamines are not recommended for treatment of pruritus associated with intrahepatic cholestasis in pregnancy (Ambros-Rudolph 2011; Kremer 2014).
Patient Education
What is this drug used for?
- It is used to relieve coughing.
- It is used to ease allergy signs.
- It is used to help motion sickness.
- It is used to treat signs like Parkinson's disease caused by other health problems.
- It is used to treat sleep problems.
Frequently reported side effects of this drug
- Fatigue
- Thickening of mucus
- Anxiety
- Vomiting
- Nausea
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Severe dizziness
- Passing out
- Decreased alertness
- Change in balance
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.