What is Fentora?
Fentora is:
- A strong prescription pain medicine that contains an opioid (narcotic) that is used to manage breakthrough pain in adults with cancer who are already routinely taking other opioid pain medicines around-the-clock for cancer pain. Fentora is started only after you have been taking other opioid pain medicines and your body has become used to them (you are opioid tolerant). Do not use Fentora if you are not opioid tolerant.
- An opioid pain medicine that can put you at risk for overdose and death. Even if you take your dose correctly as prescribed you are at risk for opioid addiction, abuse, and misuse that can lead to death.
What is the most important information I should know about Fentora?
Do not use Fentora unless you are regularly using another opioid pain medicine around-the-clock for at least one week or longer for your cancer pain and your body is used to these medicines (this means you are opioid tolerant). You can ask your healthcare provider if you are opioid tolerant.
Keep Fentora in a safe place away from children.
Get emergency help right away if:
- a child takes Fentora. Fentora can cause an overdose and death in any child who takes it.
- an adult who has not been prescribed Fentora uses it.
- an adult who is not already taking opioids around-the-clock, uses Fentora.
These are medical emergencies that can cause death. If possible, try to remove Fentora from the mouth.
Important information about Fentora:
- Get emergency help right away if you take too much Fentora (overdose). When you first start taking Fentora, when your dose is changed, or if you take too much (overdose), serious life-threatening breathing problems that can lead to death may occur.
- Taking Fentora with other medicines that may make you sleepy, such as other pain medicines, anti-depressants, sleeping pills, anti-anxiety medicines, antihistamines, or tranquilizers, or with alcohol or street drugs can cause severe drowsiness, confusion, breathing problems, coma, and death.
- Never give anyone else your Fentora. They could die from taking it. Selling or giving away Fentora is against the law.
- Store Fentora securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home.
- If you stop taking your around-the-clock opioid pain medicine for your cancer pain, you must stop using Fentora. You may no longer be opioid tolerant. Talk to your healthcare provider about how to treat your pain.
- Fentora is available only through a program called the Transmucosal Immediate Release Fentanyl (TIRF) Risk Evaluation and Mitigation Strategy (REMS) Access program. To receive Fentora, you must:
- talk to your healthcare provider
- understand the benefits and risks of Fentora
- agree to all of the instructions
- sign the Patient-Prescriber Agreement form
- Fentora is only available at pharmacies that are part of the TIRF REMS Access program. Your healthcare provider will let you know the pharmacy closest to your home where you can have your Fentora prescription filled.
- Be very careful about taking other medicines that may make you sleepy, such as other pain medicines, anti-depressant medicines, sleeping pills, anti-anxiety medicines, antihistamines, or tranquilizers.
- Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Who should not take Fentora?
Do not take Fentora if:
- You are not opioid tolerant. Opioid tolerant means that you are already taking other opioid pain medicines around-the-clock for at least one week or longer for your cancer pain, and your body is used to these medicines.
- You have severe asthma, trouble breathing, or other lung problems.
- You have a bowel blockage or have narrowing of the stomach or intestines.
- You are allergic to any of the ingredients in Fentora. See the end of this Medication Guide for a complete list of ingredients in Fentora.
- You have short-term pain that you would expect to go away in a few days, such as:
- pain after surgery
- headache or migraine
- dental pain
What should I tell my healthcare provider before taking Fentora?
Before taking Fentora, tell your healthcare provider if you have a history of:
- Troubled breathing or lung problems such as asthma, wheezing, or shortness of breath
- head injury, seizures
- slow heart rate or other heart problems
- low blood pressure
- abuse of street or prescription drugs, alcohol addiction, or mental health problems
- mental problems [including major depression, schizophrenia or hallucinations (seeing or hearing things that are not there)]
- problems urinating
- liver, kidney, thyroid problems
- pancreas or gallbladder problems
Tell your healthcare provider if you are:
- pregnant or planning to become pregnant. Prolonged use of Fentora during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated.
- breastfeeding. Fentora passes into breast milk and may harm your baby.
- taking prescription over-the-counter medicines, vitamins, or herbal supplements. Taking Fentora with certain other medicines can cause serious side effects that could lead to death.
How should I take Fentora?
When taking Fentora:
- Do not change your dose. Take Fentora exactly as prescribed by your healthcare provider.
- Your healthcare provider will change the dose until you and your healthcare provider find the right dose for you.
- See the detailed Instructions for Use at the end of this Medication Guide for information about how to use Fentora.
- Use Fentora tablets whole.
- Do not crush, split, suck, or chew Fentora tablets, or swallow the tablets whole. You will get less relief for your breakthrough cancer pain.
- Wait 30 minutes after using Fentora. If there is any of the Fentora tablet left in your mouth, you may drink a glass of water to help you swallow the left over medicine.
- You must not use more than 2 doses of Fentora for each episode of breakthrough cancer pain.
- Use 1 dose of Fentora for an episode of breakthrough cancer pain.
- If your breakthrough cancer pain does not get better 30 minutes after taking the first dose of Fentora, you can use only 1 more dose of Fentora as instructed by your healthcare provider.
- If your breakthrough pain does not get better after the second dose of Fentora, call your healthcare provider for instructions. Do not use another dose of Fentora at this time.
- Wait at least 4 hours before treating a new episode of breakthrough cancer pain with Fentora.
- If you only need to take 1 dose of Fentora for an episode of breakthrough pain, you must wait 4 hours from the time of that dose to take a dose of Fentora for a new episode of breakthrough pain.
- If you need to use 2 doses of Fentora for an episode of breakthrough pain, you must wait 4 hours after the second dose to take a dose of Fentora for a new episode of breakthrough pain.
- It is important for you to keep taking your around-the-clock opioid pain medicine while using Fentora.
- Talk to your healthcare provider if your dose of Fentora does not relieve your breakthrough cancer pain. Your healthcare provider will decide if your dose of Fentora needs to be changed.
- Talk to your healthcare provider if you have more than 4 episodes of breakthrough cancer pain per day. The dose of your around-the-clock opioid pain medicine may need to be adjusted.
- If you begin to feel dizzy, sick to your stomach, or very sleepy before the tablet is completely dissolved, rinse your mouth with water and spit the remaining pieces of the tablet into a sink or toilet right away. Rinse the sink or flush the toilet to dispose of any remaining tablet pieces.
- Do not stop taking Fentora without talking to your healthcare provider. You could become sick with uncomfortable withdrawal symptoms because your body has become used to these medicines. Physical dependency is not the same as drug addiction.
- After you stop taking, or when Fentora is no longer needed, see “How should I dispose of unused Fentora tablets when they are no longer needed?” for proper disposal of Fentora.
- Dispose of expired, unwanted, or unused Fentora by removing the product from the blister cards and promptly flushing down the toilet (if a drug take-back option is not readily available.) Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
What should I avoid while taking Fentora?
- DO NOT Drive or operate heavy machinery, until you know how Fentora affects you. Fentora can make you sleepy, dizzy, or lightheaded.
- DO NOT Drink alcohol or use prescription or over-the-counter medicines that contain alcohol. Using products containing alcohol during treatment with Fentora may cause you to overdose and die.
- DO NOT Switch from Fentora to other medicines that contain fentanyl without talking with your healthcare provider. The amount of fentanyl in a dose of Fentora is not the same as the amount of fentanyl in other medicines that contain fentanyl. Your healthcare provider will prescribe a starting dose of Fentora that may be different than other fentanyl containing medicines you may have been taking.
What are the possible side effects of Fentora?
- constipation, nausea, sleepiness, vomiting, tiredness, headache, dizziness, abdominal pain, low red blood cell count, swelling of the arms, hands, legs and feet. Call your healthcare provider if you have any of these symptoms and they are severe.
- Decreased blood pressure. This can make you feel dizzy or lightheaded if you get up too fast from sitting or lying down.
- Pain, irritation, or sores at the application site (on your gum, on the inside of your cheek, or under your tongue). Tell your healthcare provider if this is a problem for you.
Get emergency medical help if you have:
- trouble breathing, shortness of breath, fast heartbeat, chest pain, swelling of your face, tongue, or throat, extreme drowsiness, light-headedness when changing positions, feeling faint, agitation, high body temperature, trouble walking, stiff muscles, mental changes such as confusion.
- These symptoms can be a sign that you have taken too much Fentora or the dose is too high for you. These symptoms may lead to serious problems or death if not treated right away. If you have any of these symptoms, do not take any more Fentora until you have talked to your healthcare provider.
These are not all the possible side effects of Fentora. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
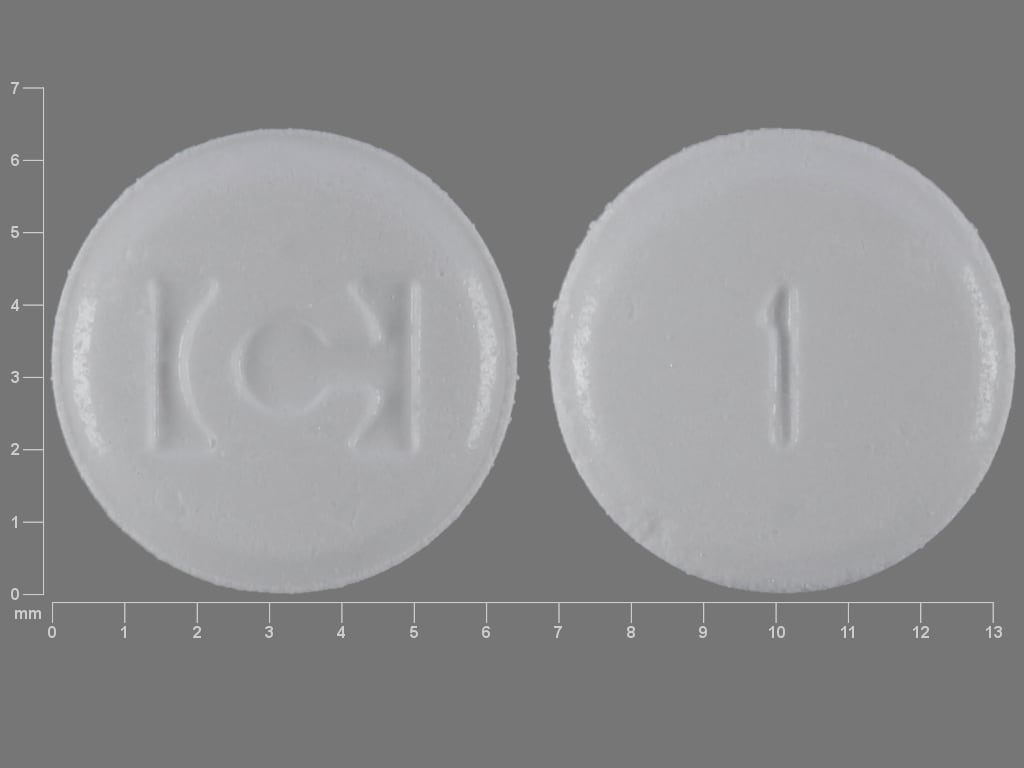
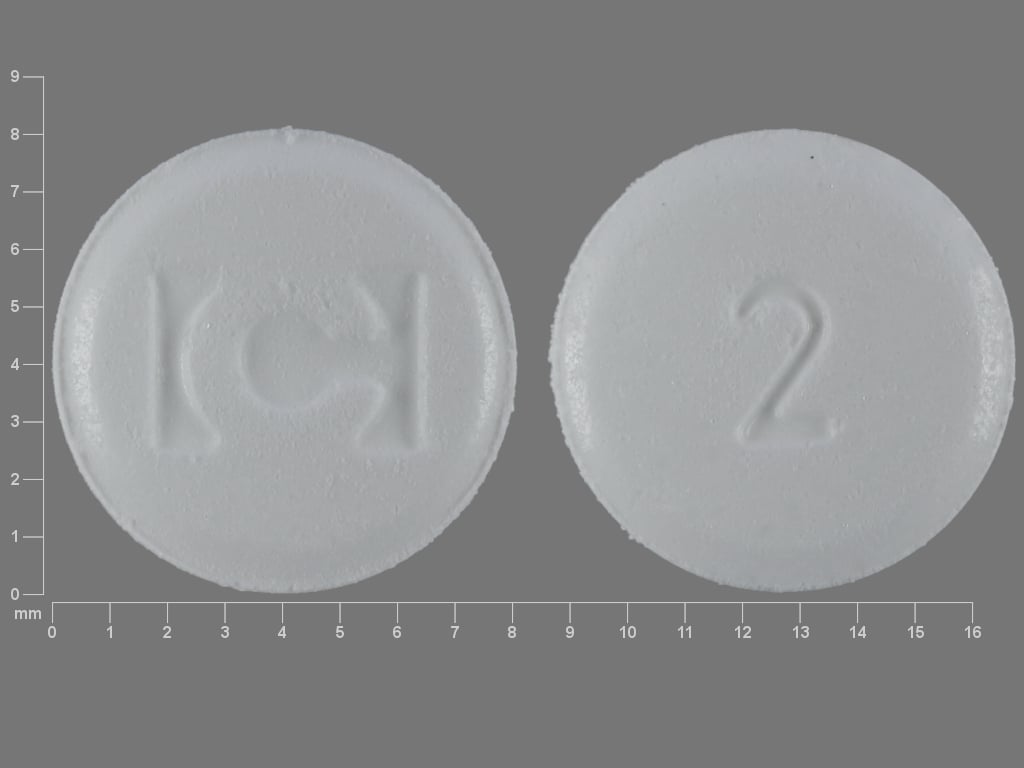
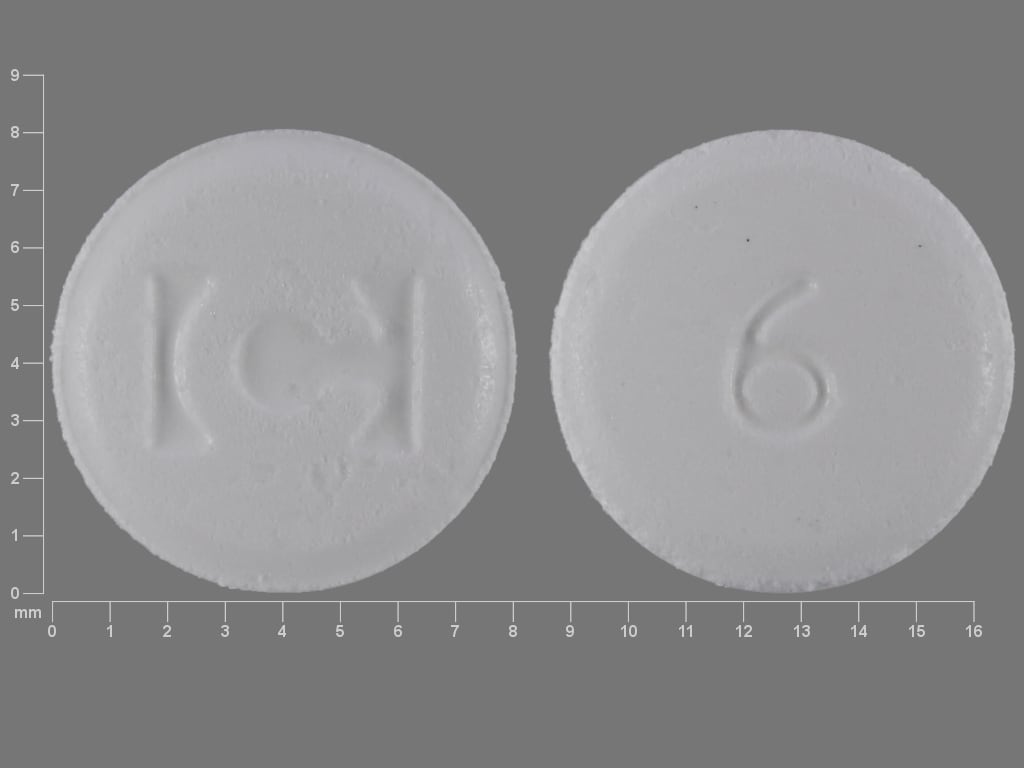
Fentora Images
General information about the safe and effective use of Fentora
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Use Fentora only for the purpose for which it was prescribed. Do not give Fentora to other people, even if they have the same symptoms you have. Fentora can harm other people and even cause death. Sharing Fentora is against the law.
This Medication Guide summarizes the most important information about Fentora. If you would like more information, talk with your healthcare provider or pharmacist. You can ask your pharmacist or healthcare provider for information about Fentora that is written for health professionals.
For more information about the TIRF REMS Access program, go to www.TIRFREMSAccess.com or call 1-866-822-1483.
How should I store Fentora?
- Always keep Fentora in a safe place away from children and from anyone for whom it has not been prescribed. Protect Fentora from theft.
- Store Fentora at room temperature, 59°F to 86°F (15° C to 30°C) until ready to use. Do not freeze Fentora.
- Keep Fentora in the original blister unit. Do not remove Fentora from its blister packaging for storage in a temporary container, such as a pill box.
- Keep Fentora dry.
How should I dispose of unused Fentora tablets when they are no longer needed?
- Dispose of any unused Fentora tablets remaining from a prescription as soon as they are no longer needed.
- Remove the tablets from blister packages and flush them down the toilet.
- Do not flush the Fentora packaging (card, blister units or cartons) down the toilet.
- If you need help with disposal of Fentora, call Teva Pharmaceuticals at 1-888-483-8279 or call your local Drug Enforcement Agency (DEA) office.
What are the ingredients in Fentora?
Active Ingredient: fentanyl citrate
Inactive Ingredients: mannitol, sodium starch glycolate, sodium bicarbonate, sodium carbonate, citric acid, and magnesium stearate.
Instructions for use
Before you use Fentora, it is important that you read the Medication Guide and these Instructions for Use. Be sure that you read, understand, and follow these Instructions for Use so that you use Fentora the right way. Ask your healthcare provider or pharmacist if you have any questions about the right way to use Fentora.
When you get an episode of breakthrough cancer pain, use the dose of Fentora prescribed by your healthcare provider as follows:
- Fentora comes packaged as a blister card containing 4 blister units. Each blister unit contains 1 Fentora tablet. Do not open a blister until ready to use.
- Separate one of the blister units from the blister card by tearing apart at the perforations. Bend the blister unit along the line where indicated. The product strength of your Fentora tablets will be printed in the boxed area shown as
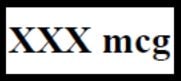
(See Figure 1).
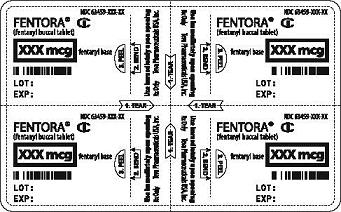
Figure 1
- Peel back foil on blister unit to expose tablet (See Figure 2).

Figure 2
- Do not push the tablet through the foil on the blister unit because this could damage the tablet.
- When removed from the blister unit, Fentora tablet must be used right away.
- Use Fentora tablets whole.
- Do not crush, split, suck, or chew Fentora tablets, or swallow the tablets whole. You will get less relief for your breakthrough cancer pain.
- You can place a Fentora tablet:
- in your mouth above a rear molar tooth between the upper cheek and gum (See Figure 3). Switch (alternate) sides of your mouth for each dose.

Figure 3
OR,
- on the floor of your mouth, under your tongue (See Figures 4a, 4b, 4c, 4d).
- When placing the tablet under your tongue, first lift your tongue (4b), then place the tablet under your tongue (4c), and lower your tongue over the tablet (4d).
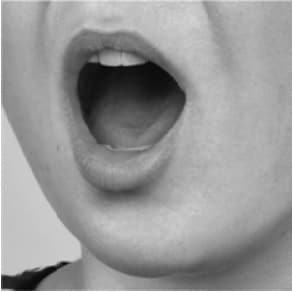
Figure 4a
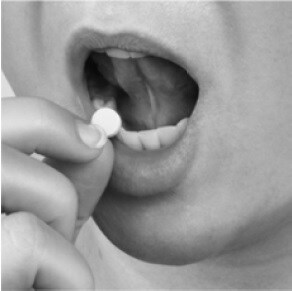
Figure 4b
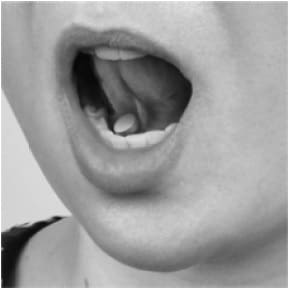
Figure 4c
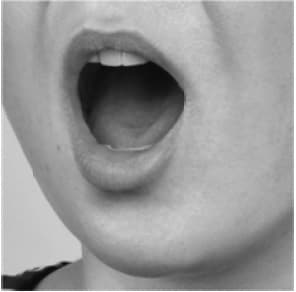
Figure 4d
- Leave the tablet in place until it dissolves. A Fentora tablet generally takes between 14 to 25 minutes to dissolve.
- After 30 minutes, if there is any Fentora left in your mouth, you may drink a glass of water to help you swallow the left over medicine.
- If you cannot use Fentora in this manner, tell your healthcare provider. Your healthcare provider will tell you what to do.