Boxed Warning
Pregnancy:
Isotretinoin must not be used by patients who are pregnant or who may become pregnant. There is an extremely high risk that severe birth defects can result if pregnancy occurs while taking isotretinoin in any amount, even for short periods of time. Potentially, any fetus exposed during pregnancy can be affected. There are no accurate means of determining whether an exposed fetus has been affected.
Birth defects that have been documented following isotretinoin exposure include abnormalities of the face, eyes, ears, skull, CNS, cardiovascular system, and thymus and parathyroid glands. Cases of intelligence quotient (IQ) scores less than 85 with or without other abnormalities have been reported. There is an increased risk of spontaneous abortion, and premature births have been reported.
Documented external abnormalities include skull abnormality; ear abnormalities (including anotia, micropinna, small or absent external auditory canals); eye abnormalities (including microphthalmia); facial dysmorphia; cleft palate. Documented internal abnormalities include CNS abnormalities (including cerebral abnormalities, cerebellar malformation, hydrocephalus, microcephaly, cranial nerve deficit); cardiovascular abnormalities; thymus gland abnormality; parathyroid hormone deficiency. In some cases, death has occurred with some of the abnormalities previously noted.
If pregnancy does occur during treatment of a patient who is taking isotretinoin, isotretinoin must be discontinued immediately and the patient should be referred to an obstetrician-gynecologist experienced in reproductive toxicity for further evaluation and counseling.
Special prescribing requirements:
Because of isotretinoin's teratogenicity and to minimize fetal exposure, isotretinoin is approved for marketing only under a special restricted distribution program approved by the Food and Drug Administration. This program is called iPLEDGE. Isotretinoin must only be prescribed by prescribers who are registered and activated with the iPLEDGE program. Isotretinoin must only be dispensed by a pharmacy registered and activated with iPLEDGE, and must only be dispensed to patients who are registered and meet all the requirements of iPLEDGE.
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Capsule, Oral:
Absorica: 10 mg, 20 mg [contains soybean oil]
Absorica: 25 mg [contains brilliant blue fcf (fd&c blue #1), fd&c yellow #6 (sunset yellow), soybean oil, tartrazine (fd&c yellow #5)]
Absorica: 30 mg [contains soybean oil]
Absorica: 35 mg [contains fd&c blue #2 (indigotine), soybean oil]
Absorica: 40 mg [contains soybean oil]
Absorica LD: 8 mg [contains brilliant blue fcf (fd&c blue #1), fd&c red #40, fd&c yellow #10 (quinoline yellow), soybean oil]
Absorica LD: 16 mg [contains brilliant blue fcf (fd&c blue #1), fd&c red #40, soybean oil]
Absorica LD: 24 mg [contains fd&c yellow #10 (quinoline yellow), fd&c yellow #6 (sunset yellow), soybean oil]
Absorica LD: 32 mg [contains soybean oil]
Amnesteem: 10 mg, 20 mg, 40 mg [contains soybean oil]
Claravis: 10 mg [contains fd&c yellow #6 (sunset yellow), soybean oil]
Claravis: 20 mg, 30 mg [contains edetate disodium, soybean oil]
Claravis: 40 mg [contains edetate disodium, fd&c yellow #6 (sunset yellow), soybean oil]
Myorisan: 10 mg, 20 mg [contains soybean oil]
Myorisan: 30 mg
Myorisan: 40 mg [contains fd&c yellow #6 (sunset yellow), soybean oil]
Zenatane: 10 mg [contains brilliant blue fcf (fd&c blue #1), edetate disodium, fd&c yellow #10 (quinoline yellow), methylparaben, propylparaben, soybean oil]
Zenatane: 20 mg [contains edetate disodium, methylparaben, propylparaben, soybean oil]
Zenatane: 30 mg [contains edetate disodium, fd&c blue #2 aluminum lake, fd&c yellow #10 (quinoline yellow), methylparaben, propylparaben, soybean oil]
Zenatane: 40 mg [contains brilliant blue fcf (fd&c blue #1), edetate disodium, fd&c blue #2 (indigotine), fd&c yellow #10 (quinoline yellow), methylparaben, propylparaben, soybean oil]
Generic: 10 mg, 20 mg, 30 mg, 40 mg
Pharmacology
Mechanism of Action
Reduces sebaceous gland size and reduces sebum production in acne treatment; in neuroblastoma, decreases cell proliferation and induces differentiation
Pharmacokinetics/Pharmacodynamics
Absorption
Absorption: Enhanced with a high-fat meal; Absorica absorption is ~83% greater than Accutane when administered under fasting conditions; they are bioequivalent when taken with a high-fat meal.
Metabolism
Hepatic via CYP2B6, 2C8, 2C9, 3A4; forms metabolites; major metabolite: 4-oxo-isotretinoin (active)
Excretion
Urine and feces (equal amounts)
Time to Peak
Serum: 3 to 5 hours
Half-Life Elimination
Terminal: Parent drug: 21 hours; Metabolite: 21 to 24 hours
Protein Binding
99% to 100%; primarily albumin
Use: Labeled Indications
Acne, severe recalcitrant nodular: Treatment of severe recalcitrant nodular acne unresponsive to conventional therapy (including systemic antibiotics)
Use: Off Label
Acne (moderate)ayes
Data from a randomized, controlled comparative study supports the use of isotretinoin (conventional and low-dose regimen) in patients with moderate acne. However, side-effects were more frequent in the conventional regimen compared to the low-dose regimen, and results suggested that the low-dose regimen had higher patient satisfaction. The authors concluded that low-dose isotretinoin is most suitable for patients with moderate acne when considering tolerability, efficacy, and patient satisfaction Lee 2011. A prospective, non-comparative, open-label study in patients with moderate acne also found low-dose isotretinoin was effective (defined as complete or almost complete remission of acne lesions) with a low incidence of severe side effects Amichai 2006.
Based on the American Academy of Dermatology guidelines of care for acne vulgaris management, isotretinoin is an effective and recommended treatment for moderate degrees of treatment-resistant acne or acne that produces physical scarring or psychological distress.
Cutaneous T-cell lymphomasc
Data from small clinical trials in patients with cutaneous T-cell lymphoma (mycosis fungoides) suggests that isotretinoin (in combination with interferon alfa-2b) may be beneficial in the treatment of this condition Duvic 2003, Knobler 1991. Additional trials may be necessary to further define the role of isotretinoin in this setting.
Squamous cell skin cancer (prevention in high-risk patients)c
Clinical experience suggests that oral retinoids may be useful for prevention of squamous cell skin cancer in high-risk patients. Acitretin may be the preferred agent in certain patient populations; however, due to a shortened half-life, isotretinoin may be preferred (with appropriate pregnancy testing and contraception) in females of reproductive potential Otley 2006. Additional data is necessary to further define the role of isotretinoin in this setting.
Contraindications
Hypersensitivity to isotretinoin or any component of the formulation; sensitivity to parabens (Zenatane only) or vitamin A; pregnancy or those who may become pregnant
Canadian labeling: Additional contraindications not in the US labeling: Breastfeeding, hepatic or renal insufficiency, hypervitaminosis A, excessive hyperlipidemia, concurrent tetracycline therapy.
Documentation of allergenic cross-reactivity for retinoids is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Dosage and Administration
Dosing: Adult
Acne (severe recalcitrant nodular):
0.5 mg/kg/day in 2 divided doses for 1 month, then increase to 1 mg/kg/day in 2 divided doses as tolerated. Continue until a total cumulative dose of 120 to 150 mg/kg is reached (AAD [Zaenglein 2016]).
Manufacturer's labeling: Dosing in the prescribing information may not reflect current clinical practice. 0.5 to 1 mg/kg/day in 2 divided doses. Adults with very severe disease/scarring or primarily involves the trunk may require dosage adjustment up to 2 mg/kg/day, as tolerated.
Acne (moderate) (off-label use): Oral: Low-dose regimens: 20 mg/day (~0.3 to 0.4 mg/kg/day) for 6 months (Amichai 2006) or 0.25 to 0.4 mg/kg daily for 24 weeks (Lee 2011)
Cutaneous T-cell lymphomas (off-label use): Oral: Induction: 1 mg/kg/day (in 2 divided doses and in combination with interferon alfa-2b) for 3 to 4 months (Duvic 2003; Knobler 1991). If response occurs, may continue therapy for an additional 3 months; if response continues after 6 months of therapy, may administer isotretinoin and interferon alfa-2b at a 50% reduced dose for an additional 3 months, followed by interferon alfa-2b maintenance therapy (Knobler 1991). Additional trials may be necessary to further define the role of isotretinoin in the management of this condition.
Squamous cell skin cancer, prevention in high-risk patients (off-label use): Oral: Initial: 0.25 mg/kg every other day for 1 month, then 0.25 mg/kg daily for one month, then 0.5 mg/kg daily. Adjust dose as needed based on tolerance; higher doses may be more effective for severe skin cancer (Otley 2006). Additional data may be necessary to further define the role of isotretinoin in this setting.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Acne vulgaris, severe recalcitrant nodular: Children ≥12 years and Adolescents: Oral: 0.5 to 1 mg/kg/day in 2 divided doses; for severe cases (involving trunk, nuchal region, lower back, buttocks, thighs) may require higher doses up to 2 mg/kg/day in 2 divided doses. Duration of therapy is typically 15 to 20 weeks or until the total cyst count decreases by 70%, whichever is sooner; an alternate reported approach is continuation until a total cumulative dose of 120 mg/kg (eg, 1 mg/kg/day for 120 days). An initial dose of ≤0.5 mg/kg/day may be used to minimize initial flaring (Strauss 2007)
Acne vulgaris, moderate: Limited data available: Children ≥12 years and Adolescents: 20 mg/day (~0.3 to 0.5 mg/kg/day) continued for 6 to 12 months to cumulative dose 120 mg/kg has been shown effective (Amichai 2006)
Neuroblastoma, maintenance: Infants, Children, and Adolescents: Oral: 80 mg/m2/dose every 12 hours for the last 2 weeks (14 consecutive days) of a 4-week cycle for 6 cycles; dinutuximab therapy is administered during the first week of the 4-week cycle (Yu 2010); begin after continuation chemotherapy or transplantation (Matthay 1999)
Extemporaneously Prepared
For patients unable to swallow the capsules whole, an oral liquid may be prepared with softgel capsules (not recommended by the manufacturers) by one of the following methods:
Place capsules (softgel formulations only) in small container and add warm (~37°C [97°F]) water or milk to cover capsule(s); wait 2 to 3 minutes until capsule is softened and then drink the milk or water with the softened capsule, or swallow softened capsule.
Puncture capsule (softgel formulations only) with needle or cut with scissors; squeeze capsule contents into 5 to 10 mL of milk or tube feed formula; draw mixture up into oral syringe and administer via feeding tube; flush feeding tube with ≥30 mL additional milk or tube feeding formula.
Puncture capsule (softgel formulations only) with needle or cut with scissors and draw contents into oral syringe; add 1 to 5 mL of medium chain triglyceride, soybean, or safflower oil to the oral syringe; mix gently and administer via feeding tube; flush feeding tube with ≥30 mL milk or tube feeding formula.
Lam MS. Extemporaneous compounding of oral liquid dosage formulations and alternative drug delivery methods for anticancer drugs. Pharmacotherapy. 2011;31(2):164-192.21275495
Administration
Oral: Administer with a meal (except Absorica, which may be taken without regard to meals). According to the manufacturer's labeling, capsules should be swallowed whole with a full glass of liquid; do not chew or suck on the capsule. For patients unable to swallow capsule whole, an oral liquid may be prepared (see Extemporaneously Prepared); may irritate esophagus if contents are removed from the capsule. Safety of once-daily dosing of isotretinoin has not been established and is not recommended.
Neuroblastoma (off-label use): In a pharmacokinetic study, the end of the capsule was punctured/cut and capsule contents extruded into ice cream or yogurt if patients were unable to swallow capsules whole; if capsule is opened, contents must be consumed immediately to avoid degradation (Veal 2007). Refer to Extemporaneously Prepared for additional information.
Dietary Considerations
Should be taken with food, except Absorica which may be taken without regard to meals. Limit intake of vitamin A; avoid use of other vitamin A products. Some formulations may contain soybean oil.
Storage
Store at 20°C to 25°C (68°F to 77° F); excursions permitted between 15°C to 30°C (59°F to 86°F). Protect from light.
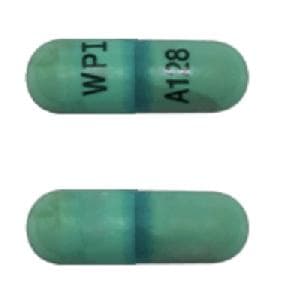
ISOtretinoin (Systemic) Images
Drug Interactions
Alcohol (Ethyl): May enhance the adverse/toxic effect of ISOtretinoin (Systemic). Specifically, the risk for elevated triglyceride concentrations may be increased. Monitor therapy
Aminolevulinic Acid (Systemic): Photosensitizing Agents may enhance the photosensitizing effect of Aminolevulinic Acid (Systemic). Avoid combination
Aminolevulinic Acid (Topical): Photosensitizing Agents may enhance the photosensitizing effect of Aminolevulinic Acid (Topical). Monitor therapy
Mipomersen: ISOtretinoin (Systemic) may enhance the hepatotoxic effect of Mipomersen. Monitor therapy
Multivitamins/Fluoride (with ADE): May enhance the adverse/toxic effect of Retinoic Acid Derivatives. Avoid combination
Multivitamins/Minerals (with ADEK, Folate, Iron): May enhance the adverse/toxic effect of Retinoic Acid Derivatives. Avoid combination
Multivitamins/Minerals (with AE, No Iron): May enhance the adverse/toxic effect of Retinoic Acid Derivatives. Avoid combination
Porfimer: Photosensitizing Agents may enhance the photosensitizing effect of Porfimer. Monitor therapy
Progestins (Contraceptive): Retinoic Acid Derivatives may diminish the therapeutic effect of Progestins (Contraceptive). Retinoic Acid Derivatives may decrease the serum concentration of Progestins (Contraceptive). Management: Two forms of effective contraception should be used in patients receiving retinoic acid derivatives. Microdosed progesterone-only preparations (ie, minipills that do not contain estrogen) are considered an inadequate method of contraception. Consider therapy modification
Tetracyclines: May enhance the adverse/toxic effect of Retinoic Acid Derivatives. The development of pseudotumor cerebri is of particular concern. Avoid combination
Verteporfin: Photosensitizing Agents may enhance the photosensitizing effect of Verteporfin. Monitor therapy
Vitamin A: May enhance the adverse/toxic effect of Retinoic Acid Derivatives. Avoid combination
Adverse Reactions
>10%:
Endocrine & metabolic: Increased serum triglycerides (25% including cases reported >800 mg/dL), increased creatine phosphokinase in blood specimen (adults: 24%; adolescents: 12%), decreased HDL cholesterol (15%)
Hepatic: Increased liver enzymes (15%)
Neuromuscular & skeletal: Back pain (children: 29%), arthralgia (≤22%), musculoskeletal disease (16%)
1% to 10%:
Endocrine & metabolic: Increased serum cholesterol (7%)
Neuromuscular & skeletal: Decreased bone mineral density (adolescents: 9%), severe arthralgia (children: 8%)
Frequency not defined:
Cardiovascular: Cerebrovascular accident, chest pain, edema, flushing, palpitations, syncope, tachycardia, thrombosis, vasculitis
Dermatologic: Acne fulminans, alopecia, cheilitis, cheilosis, contact dermatitis, dermatitis, diaphoresis, eczema, erythema of skin, facial erythema, fragile skin, hair disease, nail disease, paronychia, pruritus, pyogenic granuloma, scaling of skin of feet, seborrhea, skin photosensitivity, skin rash, sunburn, superficial peeling of palms, urticaria, xeroderma
Endocrine & metabolic: Altered serum glucose, decreased libido, eruptive xanthoma, hirsutism, increased gamma-glutamyl transferase, increased lactate dehydrogenase, increased LDL cholesterol, increased serum glucose, hyperuricemia, menstrual disease, weight loss
Gastrointestinal: Abdominal pain, colitis, constipation, decreased appetite, diarrhea, esophagitis, esophageal ulcer, gingival hemorrhage, gingivitis, ileitis, inflammatory bowel disease, nausea, pancreatitis, vomiting, xerostomia
Genitourinary: Erectile dysfunction, gross hematuria, microscopic hematuria, proteinuria, pyuria, sexual disorder
Hematologic & oncologic: Anemia, bruise, decreased white blood cell count, increased erythrocyte sedimentation rate, lymphadenopathy, purpuric disease, severe neutropenia, thrombocythemia, thrombocytopenia
Hepatic: Increased serum alanine aminotransferase, increased serum alkaline phosphatase, increased serum aspartate aminotransferase, increased serum bilirubin
Infection: Herpes simplex infection (disseminated), infection
Nervous system: Aggressive behavior, anxiety, auditory hallucinations, depression, dizziness, drowsiness, emotional lability, euphoria, fatigue, headache, idiopathic intracranial hypertension, insomnia, irritability, lethargy, malaise, nervousness, outbursts of anger, pain, panic attack, paresthesia, psychosis, seizure, suicidal ideation, suicidal tendencies, violent behavior, voice disorder
Neuromuscular & skeletal: Asthenia, arthritis, bone fracture, calcification of ligament, calcification of tendon, granulomatosis with polyangiitis, limb pain, musculoskeletal pain, myalgia, neck pain, osteopenia, osteoporosis, premature epiphyseal closure, skeletal hyperostosis, stiffness, tendonitis
Ophthalmic: Asthenopia, blepharitis, blurred vision, cataract, conjunctivitis, corneal opacity, decreased visual acuity, eye irritation, eye pruritus, hordeolum, increased lacrimation, keratitis, ocular hyperemia, optic neuritis, photophobia, vision color changes, visual disturbance
Otic: Tinnitus
Renal: Glomerulonephritis
Respiratory: Bronchospasm, dry nose, epistaxis, nasopharyngitis, respiratory tract infection, upper respiratory tract infection
Miscellaneous: Wound healing impairment
Postmarketing: Acute pancreatitis, agranulocytosis, allergic skin reaction, anaphylaxis, auditory impairment, dry eye syndrome, erythema multiforme, eyelid disease (meibomian gland dysfunction/atrophy; Neudorfer 2012), hepatitis, hypersensitivity angiitis, hypersensitivity reaction, intracranial hypertension (Tan 2019), night blindness, rhabdomyolysis, Stevens-Johnson syndrome, toxic epidermal necrolysis
Warnings/Precautions
Concerns related to adverse effects:
- Auditory impairment: Hearing impairment, which can continue after therapy is discontinued, may occur. Discontinue therapy if hearing impairment or tinnitus develops.
- Bone mineral density loss: May decrease bone mineral density; osteoporosis, osteopenia, bone fractures, and delayed healing of bone fractures have been reported. Use caution in patients with a genetic predisposition for bone loss (eg, age-related osteoporosis, history of childhood osteoporosis conditions, osteomalacia or other disorders of bone metabolism); including patients diagnosed with anorexia nervosa and those on concomitant medications that may cause drug-induced osteoporosis/osteomalacia and/or affect vitamin D metabolism (eg, systemic corticosteroids, anticonvulsants). Patients may be at increased risk when participating in activities with repetitive impact (such as sports) where the risk of spondylolisthesis with and without pars fractures and hip growth plate injuries in early and late adolescence are known.
- Dermatologic effects: Postmarketing reports of erythema multiforme severe skin reactions (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis), including fatalities, have been reported; monitor for severe skin reactions; discontinue use if severe skin reaction occurs.
- Growth effects: Skeletal hyperostosis and premature epiphyseal closure have also been reported with the use.
- Hematologic effects: Neutropenia and rare cases of agranulocytosis have been reported; discontinue if clinically significant decreases in white cell counts occur.
- Hepatic effects: Clinical hepatitis and mild to moderate elevated liver enzymes have been reported with use; liver enzymes may normalize with dosage reduction or with continued treatment. Discontinue therapy if hepatic enzymes do not normalize or if hepatitis is suspected.
- Hypersensitivity reactions: Anaphylaxis and other types of allergic reactions, including cutaneous reactions and serious cases of allergic vasculitis, often with purpura of the extremities and extracutaneous involvement (including renal) have been reported. Discontinue therapy if a serious allergic reaction occurs and institute appropriate medical management.
- Inflammatory bowel disease: Inflammatory bowel disease, including regional ileitis, has been reported in patients without a prior history of intestinal disorders; discontinue treatment immediately if abdominal pain, rectal bleeding, or severe diarrhea occurs. Of note, a position statement from the American Academy of Dermatology states that based on currently available data, there is insufficient evidence to prove either an association or a causal relationship between IBD and isotretinoin use (AAD 2016).
- Musculoskeletal effects: Musculoskeletal symptoms (including arthralgia) have been reported; generally symptoms were mild to moderate, but occasionally required discontinuation of therapy. Transient pain in the chest has occurred; symptoms generally cleared after discontinuation of therapy, but in some cases persisted. Rhabdomyolysis, some associated with strenuous physical activity, has been reported (rarely).
- Ocular effects: Vision impairment, corneal opacities, decreased tolerance to contact lenses (due to dry eyes), and decreased night vision have been reported with use; discontinue therapy in patients experiencing visual difficulties. Warn patients to be cautious when driving or operating machinery at night.
- Pancreatitis: Acute pancreatitis may occur in patients with normal or elevated triglyceride levels; fatal hemorrhagic pancreatitis (rare) has been reported; discontinue therapy if hypertriglyceridemia cannot be controlled at an acceptable level or symptoms of pancreatitis occurs.
- Photosensitivity: Avoid prolonged exposure to UV rays or sunlight.
- Pseudotumor cerebri: Retinoids have been associated with pseudotumor cerebri (benign intracranial hypertension), especially in children. Concurrent use of other drugs associated with this effect (eg, tetracyclines) may increase risk. Early signs and symptoms include papilledema, headache, nausea, vomiting, and visual disturbances; discontinue immediately and refer patient to a neurologist if papilledema occurs.
- Psychiatric effects: May cause depression, psychosis, mood disturbance, and rarely, suicidal ideation, suicide attempts, suicide, and aggressive and/or violent behaviors. All patients should be observed closely for symptoms of depression or suicidal thoughts. Discontinue therapy if depression, mood disturbance, psychosis, or aggression develops. Discontinuation of treatment alone may not be sufficient, further evaluation may be necessary. Use with extreme caution in patients with a history of psychiatric disorder.
Disease-related concerns:
- Diabetes: Use with caution in patients with diabetes mellitus; impaired glucose control has been reported.
- Hypertriglyceridemia: Marked elevations of serum triglycerides have been reported; use with caution in patients with hypertriglyceridemia or those who may be at high risk (eg, patients with diabetes, obesity, increased alcohol intake, family history of or those with lipid metabolism disorder). The effects on triglycerides, HDL, and cholesterol have been reversible upon discontinuation of therapy. Instruct patients to avoid or limit ethanol; may increase triglyceride levels if taken in excess.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pregnancy: [US Boxed Warnings]: Use of isotretinoin is contraindicated in patients who are or may become pregnant. Birth defects (facial, eye, ear, skull, central nervous system, cardiovascular, thymus and parathyroid gland abnormalities) have been noted following isotretinoin exposure during pregnancy and the risk for severe birth defects is high, with any dose or even with short treatment duration. Low IQ scores have also been reported. The risk for spontaneous abortion and premature births is increased. Because of the high likelihood of teratogenic effects, all patients (male and female), prescribers, wholesalers, and dispensing pharmacists must register and be active in the iPLEDGE risk evaluation and mitigation strategy (REMS) program; do not prescribe isotretinoin for patients who are or who are likely to become pregnant while using the drug. If pregnancy occurs during therapy, isotretinoin should be discontinued immediately and the patient referred to an obstetrician-gynecologist specializing in reproductive toxicity.
Dosage form specific issues:
- Absorica: Absorption is ~83% greater than Accutane when administered under fasting conditions; they are bioequivalent when taken with a high-fat meal. Absorica is not interchangeable with other generic isotretinoin products.
- Product interchange: Isotretinoin and tretinoin (which is also known as all-trans retinoic acid, or ATRA) may be confused, while both products may be used in cancer treatment, they are not interchangeable; verify product prior to dispensing and administration to prevent medication errors.
- Tartrazine: Some products may contain tartrazine (FD&C yellow no. 5), which may cause allergic reactions, including bronchial asthma, in certain individuals. Allergy is frequently seen in patients who also have an aspirin hypersensitivity.
Other warnings/precautions:
- Blood donation: Patients should be instructed not to donate blood during therapy and for 1 month following discontinuation of therapy due to risk of donated blood being given to a pregnant patient.
- Experienced health care provider: This medication should only be prescribed by health care providers competent in treating severe recalcitrant nodular acne and experienced with the use of systemic retinoids.
- Long-term use: Safety of long-term use is not established and is not recommended; the effect on bone loss is unknown.
- REMS program: [US Boxed Warning]: Because of the high likelihood of teratogenic effects, all patients (male and female), prescribers, wholesalers, and dispensing pharmacists must register and be active in the iPLEDGE risk evaluation and mitigation strategy (REMS) management program; do not prescribe isotretinoin for patients who are or who are likely to become pregnant while using the drug (see Additional Information or Pharmacotherapy Pearls for details). Patients of childbearing potential must be capable of complying with effective contraceptive measures. Patients must select and commit to two forms of contraception. Therapy is begun after two negative pregnancy tests; effective contraception must be used for at least 1 month before beginning therapy, during therapy, and for 1 month after discontinuation of therapy. Prescriptions should be written for no more than a 30-day supply, and pregnancy testing and counseling should be repeated monthly.
- Skin resurfacing procedures: Avoid skin resurfacing procedures (eg, dermabrasion, laser) and wax epilation during therapy and for at least 6 months after discontinuation of isotretinoin due to the risk of scarring.
Monitoring Parameters
CBC with differential and platelet count, baseline sedimentation rate, glucose, CPK; signs of depression, mood alteration, psychosis, aggression, severe skin reactions; changes in vision
Pregnancy test (for all patients of childbearing potential): Two negative tests with a sensitivity of at least 25 milliunits/mL prior to beginning therapy (the second performed at least 19 days after the first test and performed during the first 5 days of the menstrual period immediately preceding the start of therapy); monthly tests to rule out pregnancy prior to refilling prescription and one month after discontinuation (Absorica).
Lipids: Prior to treatment and at weekly or biweekly intervals until response to treatment is established. Test should not be performed <36 hours after consumption of ethanol.
Liver function tests: Prior to treatment and at weekly or biweekly intervals until response to treatment is established.
When used for oncology indications, monitor adherence.
Pregnancy
Pregnancy Risk Factor
X
Pregnancy Considerations
Isotretinoin and its metabolites can be detected in fetal tissue following maternal use during pregnancy (Benifla 1995; Kraft 1989).
Use is contraindicated in pregnant women. [US Boxed Warning]: Isotretinoin must not be used by patients who are pregnant or who may become pregnant. There is an extremely high risk that severe birth defects can result if pregnancy occurs while taking isotretinoin in any amount, even for short periods of time. Potentially, any fetus exposed during pregnancy can be affected. There are no accurate means of determining whether an exposed fetus has been affected. Birth defects that have been documented following isotretinoin exposure include abnormalities of the face, eyes, ears, skull, CNS, cardiovascular system, and thymus and parathyroid glands. Cases of intelligence quotient (IQ) scores less than 85 with or without other abnormalities have been reported. There is an increased risk of spontaneous abortion, and premature births have been reported. Documented external abnormalities include skull abnormality; ear abnormalities (including anotia, micropinna, small or absent external auditory canals); eye abnormalities (including microphthalmia); facial dysmorphia; cleft palate. Documented internal abnormalities include CNS abnormalities (including cerebral abnormalities, cerebellar malformation, hydrocephalus, microcephaly, cranial nerve deficit); cardiovascular abnormalities; thymus gland abnormality; parathyroid hormone deficiency. In some cases, death has occurred with some of the abnormalities previously noted.
If pregnancy does occur during treatment of a patient who is taking isotretinoin, isotretinoin must be discontinued immediately and the patient should be referred to an obstetrician-gynecologist experienced in reproductive toxicity for further evaluation and counseling.
Patients of childbearing potential must be able to comply with the guidelines of the iPLEDGE™ pregnancy prevention program. Females of childbearing potential must have two negative pregnancy tests with a sensitivity of at least 25 milliunits/mL prior to beginning therapy and testing should continue monthly during therapy. Patients of childbearing potential should not become pregnant during therapy or for 1 month following discontinuation of isotretinoin. Upon discontinuation of treatment, patients of childbearing potential should have a pregnancy test after their last dose and again one month after their last dose.
Two forms of contraception should be used simultaneously and continuously for ≥1 month prior to starting therapy, during therapy, and for 1 month after treatment is complete.
Any pregnancies should be reported to the iPLEDGE™ program (www.ipledgeprogram.com or 866-495-0654) and the FDA through MedWatch (800-FDA-1088).
Patient Education
What is this drug used for?
- It is used to treat pimples (acne).
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Dry mouth
- Dry eyes
- Dry skin
- Dry lips
- Nasal irritation
- Poor night vision
- Contact lens discomfort
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Severe cerebrovascular disease like change in strength on one side is greater than the other, trouble speaking or thinking, change in balance, or vision changes
- Depression like thoughts of suicide, anxiety, agitation, irritability, panic attacks, mood changes, behavioral changes, or confusion
- Infection
- Liver problems like dark urine, fatigue, lack of appetite, nausea, abdominal pain, light-colored stools, vomiting, or yellow skin.
- Esophageal problems like chest pain, trouble swallowing, or heartburn.
- High blood sugar like confusion, fatigue, increased thirst, increased hunger, passing a lot of urine, flushing, fast breathing, or breath that smells like fruit.
- Pancreatitis like severe abdominal pain, severe back pain, severe nausea, or vomiting.
- Seizures
- Behavioral changes
- Nausea
- Vomiting
- Severe headache
- Dizziness
- Severe diarrhea
- Rectal bleeding
- Rectal pain
- Severe abdominal pain
- Eye pain
- Muscle pain
- Muscle weakness
- Joint pain
- Bone pain
- Bruising
- Bleeding
- Severe loss of strength and energy
- Unable to pass urine
- Change in amount of urine passed
- Back pain
- Swelling
- Swollen gland
- Abnormal heartbeat
- Fast heartbeat
- Trouble hearing
- Noise or ringing in the ears
- Stevens-Johnson syndrome/toxic epidermal necrolysis like red, swollen, blistered, or peeling skin (with or without fever); red or irritated eyes; or sores in mouth, throat, nose, or eyes.
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.