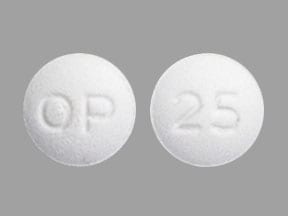
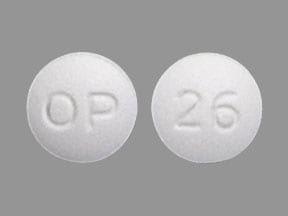
Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Glyset: 25 mg, 50 mg, 100 mg
Generic: 25 mg, 50 mg, 100 mg
Pharmacology
Mechanism of Action
In contrast to sulfonylureas, miglitol does not enhance insulin secretion; the antihyperglycemic action of miglitol results from a reversible inhibition of membrane-bound intestinal alpha-glucosidases which hydrolyze oligosaccharides and disaccharides to glucose and other monosaccharides in the brush border of the small intestine. In patients with diabetes, this enzyme inhibition results in delayed glucose absorption and lowering of postprandial hyperglycemia.
Pharmacokinetics/Pharmacodynamics
Absorption
Saturable at high doses: 25 mg dose: Completely absorbed; 100 mg dose: 50% to 70% absorbed
Distribution
Vd: 0.18 L/kg
Metabolism
None
Excretion
Urine (as unchanged drug)
Time to Peak
2-3 hours
Half-Life Elimination
~2 hours
Protein Binding
<4%
Use in Specific Populations
Special Populations: Renal Function Impairment
Because miglitol is excreted primarily by the kidneys, accumulation is expected. However, dosage adjustment to correct the increased plasma concentrations is not feasible because miglitol acts locally.
Use: Labeled Indications
Diabetes mellitus, type 2 (noninsulin-dependent, NIDDM): Adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus
Contraindications
Hypersensitivity to miglitol or any of component of the formulation; diabetic ketoacidosis; inflammatory bowel disease; colonic ulceration; partial intestinal obstruction or predisposition to intestinal obstruction; chronic intestinal diseases associated with marked disorders of digestion or absorption or with conditions that may deteriorate as a result of increased gas formation in the intestine
Dosage and Administration
Dosing: Adult
Diabetes mellitus, type 2: Oral: Initial: 25 mg 3 times daily at the start of each meal or may consider 25 mg once daily with gradual titration to 25 mg 3 times daily to minimize GI intolerance. After 4 to 8 weeks dose should be titrated to maintenance dose of 50 mg 3 times daily and continue for ~3 months; if glycosylated hemoglobin is not satisfactory, may further increase to maximum recommended dose: 100 mg 3 times daily. As tolerated, the lowest effective dose should be maintained.
Concomitant use with insulin and/or insulin secretagogues (eg, sulfonylurea): Reduced dose of insulin and/or insulin secretagogues may be needed.
Dosing: Geriatric
Refer to adult dosing.
Administration
Administer orally at the start of each main meal.
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Miglitol Images
Drug Interactions
Alpha-Lipoic Acid: May enhance the hypoglycemic effect of Antidiabetic Agents. Monitor therapy
Androgens: May enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Exceptions: Danazol. Monitor therapy
Direct Acting Antiviral Agents (HCV): May enhance the hypoglycemic effect of Antidiabetic Agents. Monitor therapy
Guanethidine: May enhance the hypoglycemic effect of Antidiabetic Agents. Monitor therapy
Hyperglycemia-Associated Agents: May diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Hypoglycemia-Associated Agents: Antidiabetic Agents may enhance the hypoglycemic effect of Hypoglycemia-Associated Agents. Monitor therapy
Maitake: May enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Monitor therapy
Monoamine Oxidase Inhibitors: May enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Monitor therapy
Pegvisomant: May enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Monitor therapy
Prothionamide: May enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Monitor therapy
Quinolones: May enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Quinolones may diminish the therapeutic effect of Blood Glucose Lowering Agents. Specifically, if an agent is being used to treat diabetes, loss of blood sugar control may occur with quinolone use. Monitor therapy
Ritodrine: May diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Salicylates: May enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Monitor therapy
Selective Serotonin Reuptake Inhibitors: May enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Monitor therapy
Thiazide and Thiazide-Like Diuretics: May diminish the therapeutic effect of Antidiabetic Agents. Monitor therapy
Adverse Reactions
>10%: Gastrointestinal: Flatulence (42%), diarrhea (29%), abdominal pain (12%)
1% to 10%: Dermatologic: Skin rash (4%)
<1%, postmarketing, and/or case reports: Abdominal distention, gastrointestinal pain, intestinal obstruction, nausea, paralytic ileus, pneumatosis cystoides intestinalis
Warnings/Precautions
Concerns related to adverse effects:
- GI symptoms: Most common reactions are gastrointestinal related; incidence of abdominal pain and diarrhea tend to diminish considerably with continued treatment.
- Hypoglycemia: Hypoglycemia is unlikely to occur with miglitol monotherapy but may occur with combination therapy (eg, sulfonylurea, insulin). In patients taking miglitol, oral glucose (dextrose) should be used instead of sucrose (cane sugar) in the treatment of mild-to-moderate hypoglycemia since the hydrolysis of sucrose to glucose and fructose is inhibited by miglitol. Correction of severe hypoglycemia may require the use of either glucagon or IV glucose.
Disease-related concerns:
- Renal impairment: Not recommended in severe impairment (serum creatinine >2 mg/dL or CrCl <25 mL/minute).
- Stress-related states: It may be necessary to discontinue miglitol and administer insulin if the patient is exposed to stress (ie, fever, trauma, infection, surgery).
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Monitoring Parameters
Monitor therapeutic response by periodic blood glucose tests; hemoglobin A1c (at least twice yearly in patients who have stable glycemic control and are meeting treatment goals; quarterly in patients not meeting treatment goals or with therapy change [ADA 2019]).
Pregnancy
Pregnancy Considerations
Poorly controlled diabetes during pregnancy can be associated with an increased risk of adverse maternal and fetal outcomes, including diabetic ketoacidosis, preeclampsia, spontaneous abortion, preterm delivery, delivery complications, major birth defects, stillbirth, and macrosomia (ACOG 201 2018). To prevent adverse outcomes, prior to conception and throughout pregnancy, maternal blood glucose and HbA1c should be kept as close to target goals as possible but without causing significant hypoglycemia (ADA 2020; Blumer 2013).
Agents other than miglitol are currently recommended to treat diabetes mellitus in pregnancy (ADA 2020).
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience abdominal pain, passing gas, or diarrhea. Have patient report immediately to prescriber signs of low blood sugar (dizziness, headache, fatigue, feeling weak, shaking, a fast heartbeat, confusion, hunger, or sweating) (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for healthcare professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience and judgment in diagnosing, treating and advising patients.