Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Solution, Nasal:
Synarel: 2 mg/mL (8 mL)
Pharmacology
Mechanism of Action
Potent synthetic decapeptide analogue of gonadotropin-releasing hormone (GnRH; LHRH) which is approximately 200 times more potent than GnRH in terms of pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Effects on the pituitary gland and sex hormones are dependent upon its length of administration. After acute administration, an initial stimulation of the release of LH and FSH from the pituitary is observed; an increase in androgens and estrogens subsequently follows. Continued administration of nafarelin, however, suppresses gonadotrope responsiveness to endogenous GnRH resulting in reduced secretion of LH and FSH and, secondarily, decreased ovarian and testicular steroid production.
Pharmacokinetics/Pharmacodynamics
Metabolism
Degraded by peptidase; forms metabolites
Excretion
Urine (44% to 55%, ~3% as unchanged drug); feces (19% to 44%)
Time to Peak
Serum: 10 to 45 minutes
Half-Life Elimination
~3 hours; Metabolites: ~86 hours
Protein Binding
Plasma: ~80%
Use: Labeled Indications
Central precocious puberty: Treatment of central precocious puberty (CPP) (gonadotropin-dependent precocious puberty) in children of both sexes.
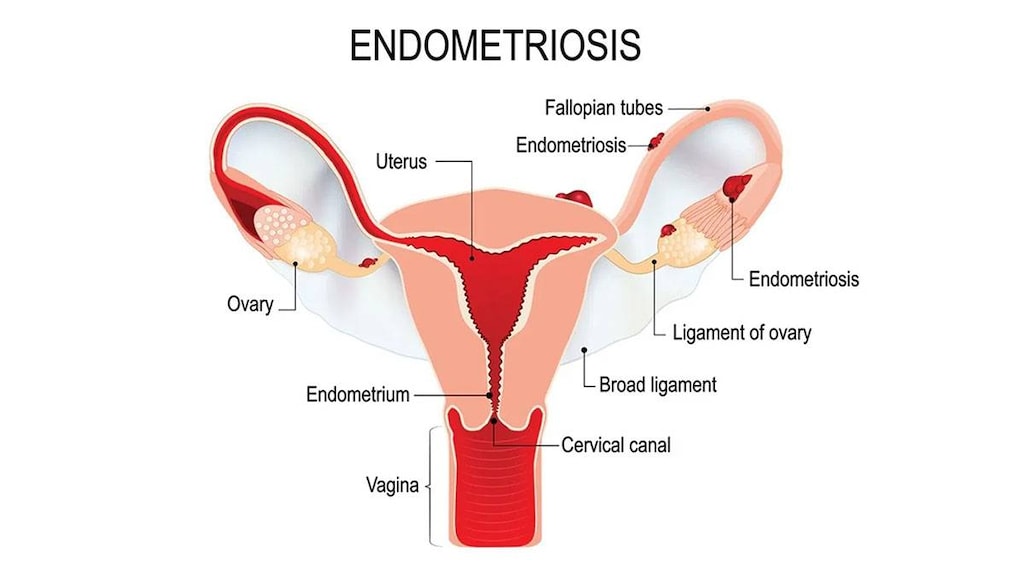
Endometriosis: Management of endometriosis, including pain relief and reduction of endometriotic lesions.
Contraindications
Hypersensitivity to gonadotropin-releasing hormone (GnRH), GnRH-agonist analogs, or any component of the formulation; undiagnosed abnormal vaginal bleeding; pregnant women or those who may become pregnant; breast-feeding
Dosage and Administration
Dosing: Adult
Endometriosis: Intranasal: Females: One spray (200 mcg) into 1 nostril each morning and 1 spray (200 mcg) into the other nostril each evening starting between days 2 and 4 of menstrual cycle (total: 2 sprays [400 mcg] daily). If regular menstruation persists after 2 months of therapy, may increase dose to 2 sprays (400 mcg; 1 spray in each nostril) in the morning and evening (total: 4 sprays [800 mcg] daily). Total duration of therapy should not exceed 6 months due to decreases in bone mineral density; retreatment is not recommended by the manufacturer.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Central precocious puberty: Intranasal: Males/Females: (US labeling): Two sprays (400 mcg) into each nostril in the morning and 2 sprays (400 mcg) into each nostril in the evening (total: 8 sprays [1,600 mcg] daily). If inadequate suppression, may increase dose to 3 sprays (600 mcg) into alternating nostrils 3 times daily (total: 9 sprays [1,800 mcg] daily). Continue therapy until resumption of puberty is desired.
Administration
Nasal spray: Allow ~30 seconds to elapse between sprays. Sneezing during or immediately after dosing should be avoided (may decrease drug absorption). Do not use a topical nasal decongestant for at least 2 hours (US labeling) or at least 30 minutes (Canadian labeling) after nafarelin use.
Storage
Store at 15°C to 30°C (59°F to 86°F). Protect from light. Do not freeze.
Drug Interactions
Choline C 11: Luteinizing Hormone-Releasing Hormone Analogs may diminish the therapeutic effect of Choline C 11. Monitor therapy
Corifollitropin Alfa: Luteinizing Hormone-Releasing Hormone Analogs may enhance the therapeutic effect of Corifollitropin Alfa. Avoid combination
Indium 111 Capromab Pendetide: Luteinizing Hormone-Releasing Hormone Analogs may diminish the diagnostic effect of Indium 111 Capromab Pendetide. Avoid combination
Test Interactions
Diagnostic tests of pituitary gonadotropic and gonadal functions during and up to 4 to 8 weeks after discontinuing treatment may be misleading.
Adverse Reactions
Adverse events may be more frequent in the first 6 weeks of treatment due to stimulation of the pituitary-gonadal axis.
CPP: 1% to 10%:
Central nervous system: Emotional lability (6%)
Dermatologic: Acne vulgaris (10%), hypertrichosis (transient, pubic region: 5%), body odor (4%), seborrhea (3%)
Endocrine & metabolic: Breast hypertrophy (8%; transient), vaginal hemorrhage (8%), hot flashes (3%; transient), vaginal discharge (3%)
Hypersensitivity: Hypersensitivity reaction (3%; including chest pain, pruritus, dyspnea, skin rash)
Respiratory: Rhinitis (5%)
Endometriosis:
>10%:
Central nervous system: Headache (18%), emotional lability (16%)
Dermatologic: Acne vulgaris (14%)
Endocrine & metabolic: Hot flash (90%), decreased libido (23%), hyperphosphatemia (10% to 15%), hypocalcemia (10% to 15%), hypertriglyceridemia (12%)
Genitourinary: Vaginal dryness (19%)
Hematologic & oncologic: Change in WBC count (10% to 15%; decreased), eosinophilia (10% to 15%)
1% to 10%:
Cardiovascular: Edema (8%)
Central nervous system: Insomnia (8%), depression (3%)
Dermatologic: Hirsutism (3%)
Endocrine & metabolic: Breast atrophy (10%), hypercholesterolemia (6%), increased libido (2%)
Gastrointestinal: Weight gain (8%), weight loss (2%)
Neuromuscular & skeletal: Myalgia (10%), decreased bone mineral density
Respiratory: Nasal mucosa irritation (10%)
<1%, postmarketing, and/or case reports (any indication): Arterial thromboembolism, arthralgia, breast engorgement, chloasma, eye pain, hepatic injury, increased serum ALT, increased serum AST, lactation, maculopapular rash, palpitations, paresthesia, pituitary apoplexy, seizures, venous thromboembolism, weakness
Warnings/Precautions
Concerns related to adverse effects:
- Decreased bone density: Has been reported and may be irreversible. Use with caution in patients with risk factors for bone loss (eg, chronic alcohol use, anticonvulsant or corticosteroid therapy, family history of osteoporosis). Repeat courses are not recommended in patients with major risk factors for bone loss.
- Ovarian cysts: May occur within the first 2 months of therapy and may occur more commonly in women with polycystic ovarian disease. These cysts may resolve spontaneously, generally by about 4 to 6 weeks of therapy, but sometimes require discontinuation of therapy and/or surgical intervention.
- Pituitary apoplexy: Rare cases of pituitary apoplexy (frequently secondary to pituitary adenoma) have been observed with GnRH agonist administration (onset from 1 hour to usually <2 weeks); may present as sudden headache, vomiting, visual or mental status changes, and infrequently cardiovascular collapse; immediate medical attention may be required.
- Psychiatric events: Emotional lability, such as crying, irritability, impatience, anger, and aggression, has been reported in patients taking GnRH agonists. Depression, including rare reports of suicidal ideation and attempt, has been reported for GnRH agonists in children treated for CPP; many of these patients had a history of psychiatric illness or other comorbidities with an increased risk of depression. Monitor for development or worsening of psychiatric symptoms, including depression, during treatment.
- Seizures: Have been observed in patients receiving GnRH agonists; patients with a history of seizures, cerebrovascular disorders, CNS anomalies/tumors, and patients on concomitant medications that have been associated with seizures are at increased risk; has also been reported in patients without risk factors.
Disease-related concerns:
- CPP use: When used for the treatment of CPP, some signs of puberty (eg vaginal bleeding, breast enlargement) may occur but should resolve within the first 2 months of therapy.
Other warnings/precautions:
- Appropriate use: Endometriosis: Experience with nafarelin for the management of endometriosis has been limited to women 18 years and older treated for 6 months; retreatment is not recommended since safety data is not available for use >6 months.
Monitoring Parameters
CPP: Bone mineral density, GnRH testing (blood LH and FSH levels), measurement of bone age, Tanner staging; monitor for development or worsening of psychiatric symptoms, including depression.
Endometriosis: Menstruation, vaginal bleeding or spotting which persists after 2 months of treatment
Pregnancy
Pregnancy Considerations
Use is contraindicated in pregnant women and pregnancy should be excluded prior to initiating treatment. Ovulation is inhibited and menstruation is stopped when used appropriately for the treatment of endometriosis; however, contraception is not assured. Nonhormonal contraception is recommended. There is no evidence that pregnancy rates are enhanced or adversely affected by use.
Patient Education
- Discuss specific use of drug and side effects with patient as it relates to treatment. (HCAHPS: During this hospital stay, were you given any medicine that you had not taken before? Before giving you any new medicine, how often did hospital staff tell you what the medicine was for? How often did hospital staff describe possible side effects in a way you could understand?)
- Patient may experience headache, hot flashes, acne, muscle pain, weight gain, vaginal dryness, runny nose, trouble sleeping, decreased sex drive, enlarged breasts, change in body odor, or signs of puberty. Have patient report immediately to prescriber chest pain, severe stuffy nose, menstruation, mood changes, behavioral changes, shortness of breath, abdominal pain, swelling of arms or legs, vaginal discharge, abnormal vaginal bleeding, bone pain, seizures, or signs of pituitary apoplexy (sudden headache, vomiting, passing out, mood changes, eye weakness, unable to move eyes, or vision changes) (HCAHPS).
- Educate patient about signs of a significant reaction (eg, wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat). Note: This is not a comprehensive list of all side effects. Patient should consult prescriber for additional questions.
Intended Use and Disclaimer: Should not be printed and given to patients. This information is intended to serve as a concise initial reference for health care professionals to use when discussing medications with a patient. You must ultimately rely on your own discretion, experience, and judgment in diagnosing, treating, and advising patients.