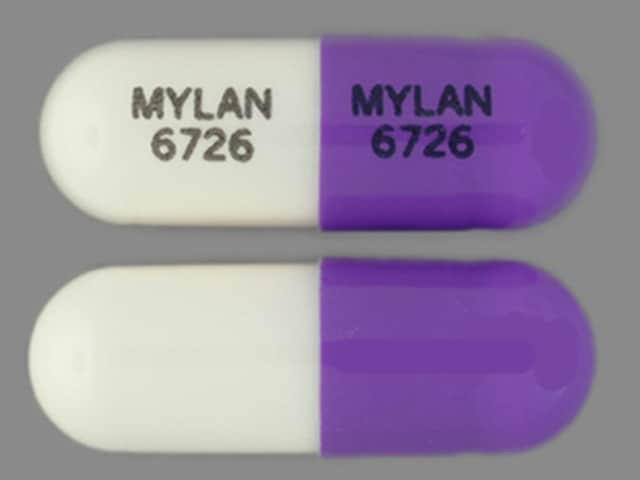
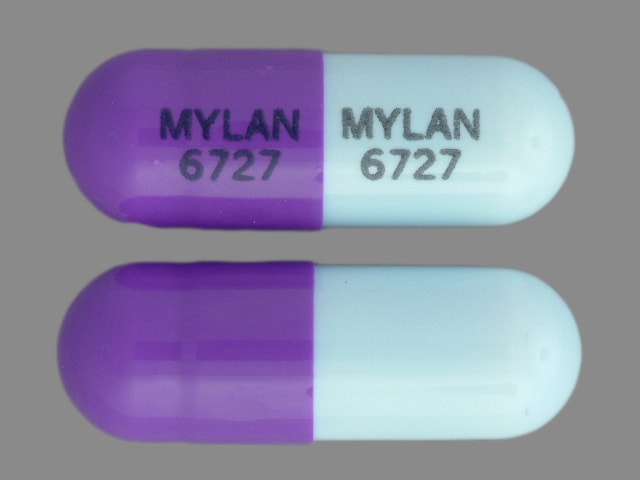
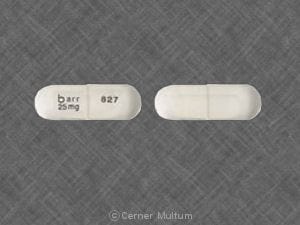
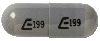Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling. [DSC] = Discontinued product
Capsule, Oral:
Zonegran: 25 mg, 100 mg [DSC]
Zonegran: 100 mg [contains fd&c red #40, fd&c yellow #6 (sunset yellow)]
Generic: 25 mg, 50 mg, 100 mg
Pharmacology
Mechanism of Action
Stabilizes neuronal membranes and suppresses neuronal hypersynchronization through action at sodium and calcium channels; does not affect GABA activity.
Pharmacokinetics/Pharmacodynamics
Absorption
Oral: Rapid and complete
Distribution
Vd: 1.45 L/kg
Metabolism
Hepatic via CYP3A4; undergoes acetylation to form N-acetyl zonisamide and reduction via cytochrome P450 isoenzyme CYP3A4 to 2-sulfamoylacetylphenol (SMAP); SMAP then undergoes conjugation with glucuronide
Excretion
Urine (62%, 35% as unchanged drug, 65% as metabolites); feces (3%); Note: Of the dose recovered in Japanese patients, 28% is as unchanged drug, 52% is as N-acetyl zonisamide, and 19% is as SMAP glucuronide (Glauser 2002)
Time to Peak
2 to 6 hours
Half-Life Elimination
Plasma: ~63 hours (range: 50 to 68 hours)
Protein Binding
40%
Use in Specific Populations
Special Populations: Renal Function Impairment
Renal clearance decreases with decreased renal function. Marked renal impairment (CrCl less than 20 mL/minute) was associated with an increase in AUC of 35%.
Use: Labeled Indications
Focal (partial) onset seizures: Adjunctive therapy in the treatment of focal onset seizures in adolescents >16 years of age and adults.
Use: Off Label
Binge eating disordercyes
Data from a limited number of controlled and noncontrolled studies suggest that zonisamide may reduce binge eating episodes and body weight in obese patients with binge eating disorder; however, zonisamide may be poorly tolerated McElroy 2004, McElroy 2006, Ricca 2009.
Based on the American Psychiatric Association guidelines for the treatment of patients with eating disorders and the World Federation of Societies of Biological Psychiatry guidelines for the pharmacological treatment of eating disorders, zonisamide may be considered for the treatment of binge eating disorder
Contraindications
Hypersensitivity to zonisamide, sulfonamides, or any component of the formulation
Note: Although the FDA approved product labeling states this medication is contraindicated with other sulfonamide-containing drug classes, the scientific basis of this statement has been challenged. See “Warnings/Precautions” for more detail.
Dosage and Administration
Dosing: Adult
Binge-eating disorder (off-label use): Oral: Initial: 100 mg/day for 7 days; may increase dose based on response and tolerability in increments of 100 mg/day every week to a maximum dose of 600 mg/day in 1 or 2 divided doses (McElroy 2004; McElroy 2006). Alternatively, 25 mg/day for 7 days has been studied; may increase dose based on response and tolerability in increments of 50 mg/day every week to a maximum daily dose of 100 mg in patients with a BMI <35 kg/m2 or 150 mg for a BMI ≥35 kg/m2 (Ricca 2009).
Focal (partial) onset seizures: Note: FDA approved for adjunctive treatment of focal onset seizures; may be used off label as monotherapy for focal onset seizures (Baulac 2012; Baulac 2014): Oral: Initial: 100 mg/day. Dose may be increased to 200 mg/day after 2 weeks. Further dosage increases to 300 and 400 mg/day can then be made with a minimum of 2 weeks between adjustments, in order to reach steady state at each dosage level. Doses of up to 600 mg/day have been studied; however, there is no evidence of increased response with doses >400 mg/day, and doses ≥300 mg/day are associated with increased side effects.
Discontinuation of zonisamide therapy: In chronic therapy, withdraw gradually to minimize the potential of increased seizure frequency (in patients with epilepsy) and withdrawal symptoms (eg, dysphoria, hallucinations, headache, insomnia, tremor), unless safety concerns require more rapid withdrawal. For seizure disorders, some experts suggest withdrawing antiepileptic drugs over a few to several (eg, 2 to 6) months (Schachter 2019).
Dosing: Geriatric
Data from clinical trials is insufficient for patients older than 65. Begin dosing at the low end of the dosing range.
Dosing: Pediatric
Partial seizures; adjunct therapy:
Infants, Children, and Adolescents ≤16 years: Limited data available; dosing regimens variable: Initial: 1 to 2 mg/kg/day given in two divided doses/day; increase dose in increments of 0.5 to 1 mg/kg/day every 2 weeks; usual dose: 5 to 8 mg/kg/day; dosing based on a review article of the literature (primarily Japanese experience) (Glauser 2002).Others have recommended higher initial and maximum doses: Initial: 2 to 4 mg/kg/day given in two divided doses/day; titrate dose upwards if needed every 2 weeks; usual dose: 4 to 8 mg/kg/day; maximum daily dose: 12 mg/kg/day (Leppik 1999; Oommen 1999)
Adolescents >16 years: Initial: 100 mg once daily; dose may be increased to 200 mg/day after 2 weeks; further increases in dose should be made in increments of 100 mg/day and only after a minimum of 2 weeks between adjustments; usual effective dose: 100 to 600 mg/day. Note: There is no evidence of increased benefit with doses >400 mg/day. Steady-state serum concentrations fluctuate 27% with once daily dosing, and 14% with twice daily dosing; patients may benefit from divided doses given twice daily (Leppik 1999)
Infantile spasms: Limited data available: Faster titration of zonisamide has been used to control infantile spasms. Suzuki treated 11 newly diagnosed infants (mean age: ~6 months) with zonisamide monotherapy starting at doses of 3 to 5 mg/kg/day given in 2 divided doses/day; doses were increased every 4th day until seizures were controlled or a maximum dose of 10 mg/kg/day was attained; four of 11 patients responded at doses of 4 to 5 mg/kg/day (Suzuki 1997). Yanai treated 27 newly diagnosed infantile spasm patients with zonisamide add-on or monotherapy starting at doses of 2 to 4 mg/kg/day given in 2 divided doses/day; doses were increased by 2 to 5 mg/kg every 2 to 4 days until seizures were controlled or a maximum of 10 to 20 mg/kg/day was attained; nine of 27 patients responded at a mean effective dose of 7.8 mg/kg/day (range: 5 to 12.5 mg/kg/day); non-responders received doses of 8 to 20 mg/kg/day (mean: 10.8 mg/kg/day) (Yania 1999). Suzuki treated 54 newly diagnosed infants (11 of which were previously reported) with zonisamide monotherapy starting at doses of 3 to 4 mg/kg/day given in 2 divided doses; doses were increased every 4th day until seizures were controlled or a maximum dose of 10 to 13 mg/kg/day was attained; 11 of 54 infants responded at a mean effective dose of 7.2 mg/kg/day (range: 4 to 12 mg/kg/day); the majority of infants who responded did so at a dose of 4 to 8 mg/kg/day and within 1 to 2 weeks of starting therapy (Suzuki 2001).
Extemporaneously Prepared
10 mg/mL Oral Suspension (ASHP Standard Concentration) (ASHP 2017)
A 10 mg/mL suspension may be made using capsules and either simple syrup or methylcellulose 0.5%. Empty contents of ten 100 mg capsules into glass mortar. Reduce to a fine powder and add a small amount of Simple Syrup, NF and mix to a uniform paste; mix while adding the chosen vehicle in incremental proportions to almost 100 mL; transfer to an amber calibrated plastic bottle, rinse mortar with vehicle, and add quantity of vehicle sufficient to make 100 mL. Label "shake well" and "refrigerate." When using simple syrup vehicle, stable 28 days at room temperature or refrigerated (preferred). When using methylcellulose vehicle, stable 7 days at room temperature or 28 days refrigerated. Note: Although no visual evidence of microbial growth was observed, storage under refrigeration would be recommended to minimize microbial contamination.
Abobo CV, Wei B, and Liang D, "Stability of Zonisamide in Extemporaneously Compounded Oral Suspensions," Am J Health Syst Pharm, 2009, 66(12):1105-9.19498126
Administration
Capsules should be swallowed whole. Administer once or twice daily with or without food.
Storage
Store at 25°C (77°F) excursions are permitted between 15°C and 30°C (59°F and 86°F). Protect from moisture and light.
Zonisamide Images
Drug Interactions
Alcohol (Ethyl): CNS Depressants may enhance the CNS depressant effect of Alcohol (Ethyl). Monitor therapy
Alizapride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Alpha-/Beta-Agonists (Indirect-Acting): Carbonic Anhydrase Inhibitors may increase the serum concentration of Alpha-/Beta-Agonists (Indirect-Acting). Monitor therapy
Amantadine: Carbonic Anhydrase Inhibitors may increase the serum concentration of Amantadine. Monitor therapy
Amphetamines: Carbonic Anhydrase Inhibitors may decrease the excretion of Amphetamines. Monitor therapy
Azelastine (Nasal): CNS Depressants may enhance the CNS depressant effect of Azelastine (Nasal). Avoid combination
Blonanserin: CNS Depressants may enhance the CNS depressant effect of Blonanserin. Consider therapy modification
Bosentan: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Brexanolone: CNS Depressants may enhance the CNS depressant effect of Brexanolone. Monitor therapy
Brimonidine (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Bromopride: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Bromperidol: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
Buprenorphine: CNS Depressants may enhance the CNS depressant effect of Buprenorphine. Management: Consider reduced doses of other CNS depressants, and avoiding such drugs in patients at high risk of buprenorphine overuse/self-injection. Initiate buprenorphine at lower doses in patients already receiving CNS depressants. Consider therapy modification
Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Cannabis: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Carbonic Anhydrase Inhibitors: May enhance the adverse/toxic effect of other Carbonic Anhydrase Inhibitors. The development of acid-base disorders with concurrent use of ophthalmic and oral carbonic anhydrase inhibitors has been reported. Management: Avoid concurrent use of different carbonic anhydrase inhibitors if possible. Monitor patients closely for the occurrence of kidney stones and with regards to severity of metabolic acidosis. Avoid combination
Chlormethiazole: May enhance the CNS depressant effect of CNS Depressants. Management: Monitor closely for evidence of excessive CNS depression. The chlormethiazole labeling states that an appropriately reduced dose should be used if such a combination must be used. Consider therapy modification
Chlorphenesin Carbamate: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
CNS Depressants: May enhance the adverse/toxic effect of other CNS Depressants. Monitor therapy
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May increase the metabolism of CYP3A4 Substrates (High risk with Inducers). Management: Consider an alternative for one of the interacting drugs. Some combinations may be specifically contraindicated. Consult appropriate manufacturer labeling. Consider therapy modification
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Dimethindene (Topical): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Doxylamine: May enhance the CNS depressant effect of CNS Depressants. Management: The manufacturer of Diclegis (doxylamine/pyridoxine), intended for use in pregnancy, specifically states that use with other CNS depressants is not recommended. Monitor therapy
Dronabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Droperidol: May enhance the CNS depressant effect of CNS Depressants. Management: Consider dose reductions of droperidol or of other CNS agents (eg, opioids, barbiturates) with concomitant use. Exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Consider therapy modification
Enzalutamide: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Concurrent use of enzalutamide with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Use of enzalutamide and any other CYP3A4 substrate should be performed with caution and close monitoring. Consider therapy modification
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Esketamine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Flecainide: Carbonic Anhydrase Inhibitors may increase the serum concentration of Flecainide. Monitor therapy
Flunitrazepam: CNS Depressants may enhance the CNS depressant effect of Flunitrazepam. Consider therapy modification
Fosphenytoin: May decrease the serum concentration of Zonisamide. Monitor therapy
HYDROcodone: CNS Depressants may enhance the CNS depressant effect of HYDROcodone. Management: Avoid concomitant use of hydrocodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
HydrOXYzine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Ivosidenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Kava Kava: May enhance the adverse/toxic effect of CNS Depressants. Monitor therapy
Lacosamide: Antiepileptic Agents (Sodium Channel Blockers) may enhance the adverse/toxic effect of Lacosamide. Specifically the risk for bradycardia, ventricular tachyarrhythmias, or a prolonged PR interval may be increased. Monitor therapy
Lemborexant: May enhance the CNS depressant effect of CNS Depressants. Management: Dosage adjustments of lemborexant and of concomitant CNS depressants may be necessary when administered together because of potentially additive CNS depressant effects. Close monitoring for CNS depressant effects is necessary. Consider therapy modification
Lithium: Carbonic Anhydrase Inhibitors may decrease the serum concentration of Lithium. Monitor therapy
Lofexidine: May enhance the CNS depressant effect of CNS Depressants. Management: Drugs listed as exceptions to this monograph are discussed in further detail in separate drug interaction monographs. Monitor therapy
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
Magnesium Sulfate: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Mefloquine: May diminish the therapeutic effect of Anticonvulsants. Mefloquine may decrease the serum concentration of Anticonvulsants. Management: Mefloquine is contraindicated for malaria prophylaxis in persons with a history of convulsions. Monitor anticonvulsant concentrations and treatment response closely with concurrent use. Consider therapy modification
Memantine: Carbonic Anhydrase Inhibitors may increase the serum concentration of Memantine. Monitor therapy
MetFORMIN: Carbonic Anhydrase Inhibitors may enhance the adverse/toxic effect of MetFORMIN. Specifically, the risk of developing lactic acidosis may be increased. Monitor therapy
Methenamine: Carbonic Anhydrase Inhibitors may diminish the therapeutic effect of Methenamine. Management: Consider avoiding this combination. Monitor for decreased therapeutic effects of methenamine if used concomitant with a carbonic anhydrase inhibitor. Consider therapy modification
Methotrimeprazine: CNS Depressants may enhance the CNS depressant effect of Methotrimeprazine. Methotrimeprazine may enhance the CNS depressant effect of CNS Depressants. Management: Reduce adult dose of CNS depressant agents by 50% with initiation of concomitant methotrimeprazine therapy. Further CNS depressant dosage adjustments should be initiated only after clinically effective methotrimeprazine dose is established. Consider therapy modification
MetyroSINE: CNS Depressants may enhance the sedative effect of MetyroSINE. Monitor therapy
Mianserin: May diminish the therapeutic effect of Anticonvulsants. Monitor therapy
Minocycline (Systemic): May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Mitotane: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Doses of CYP3A4 substrates may need to be adjusted substantially when used in patients being treated with mitotane. Consider therapy modification
Nabilone: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Opioid Agonists: CNS Depressants may enhance the CNS depressant effect of Opioid Agonists. Management: Avoid concomitant use of opioid agonists and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Orlistat: May decrease the serum concentration of Anticonvulsants. Monitor therapy
Orphenadrine: CNS Depressants may enhance the CNS depressant effect of Orphenadrine. Avoid combination
Oxomemazine: May enhance the CNS depressant effect of CNS Depressants. Avoid combination
OxyCODONE: CNS Depressants may enhance the CNS depressant effect of OxyCODONE. Management: Avoid concomitant use of oxycodone and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Paraldehyde: CNS Depressants may enhance the CNS depressant effect of Paraldehyde. Avoid combination
Perampanel: May enhance the CNS depressant effect of CNS Depressants. Management: Patients taking perampanel with any other drug that has CNS depressant activities should avoid complex and high-risk activities, particularly those such as driving that require alertness and coordination, until they have experience using the combination. Consider therapy modification
PHENobarbital: May decrease the serum concentration of Zonisamide. Monitor therapy
Phenytoin: May decrease the serum concentration of Zonisamide. Monitor therapy
Piribedil: CNS Depressants may enhance the CNS depressant effect of Piribedil. Monitor therapy
Pramipexole: CNS Depressants may enhance the sedative effect of Pramipexole. Monitor therapy
QuiNIDine: Carbonic Anhydrase Inhibitors may decrease the excretion of QuiNIDine. Monitor therapy
ROPINIRole: CNS Depressants may enhance the sedative effect of ROPINIRole. Monitor therapy
Rotigotine: CNS Depressants may enhance the sedative effect of Rotigotine. Monitor therapy
Rufinamide: May enhance the adverse/toxic effect of CNS Depressants. Specifically, sleepiness and dizziness may be enhanced. Monitor therapy
Salicylates: May enhance the adverse/toxic effect of Carbonic Anhydrase Inhibitors. Salicylate toxicity might be enhanced by this same combination. Management: Avoid these combinations when possible.Dichlorphenamide use with high-dose aspirin as contraindicated. If another combination is used, monitor patients closely for adverse effects. Tachypnea, anorexia, lethargy, and coma have been reported. Consider therapy modification
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Selective Serotonin Reuptake Inhibitors: CNS Depressants may enhance the adverse/toxic effect of Selective Serotonin Reuptake Inhibitors. Specifically, the risk of psychomotor impairment may be enhanced. Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Sodium Oxybate: May enhance the CNS depressant effect of CNS Depressants. Management: Consider alternatives to combined use. When combined use is needed, consider minimizing doses of one or more drugs. Use of sodium oxybate with alcohol or sedative hypnotics is contraindicated. Consider therapy modification
Suvorexant: CNS Depressants may enhance the CNS depressant effect of Suvorexant. Management: Dose reduction of suvorexant and/or any other CNS depressant may be necessary. Use of suvorexant with alcohol is not recommended, and the use of suvorexant with any other drug to treat insomnia is not recommended. Consider therapy modification
Tapentadol: May enhance the CNS depressant effect of CNS Depressants. Management: Avoid concomitant use of tapentadol and benzodiazepines or other CNS depressants when possible. These agents should only be combined if alternative treatment options are inadequate. If combined, limit the dosages and duration of each drug. Consider therapy modification
Tetrahydrocannabinol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Tetrahydrocannabinol and Cannabidiol: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Thalidomide: CNS Depressants may enhance the CNS depressant effect of Thalidomide. Avoid combination
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Trimeprazine: May enhance the CNS depressant effect of CNS Depressants. Monitor therapy
Zolpidem: CNS Depressants may enhance the CNS depressant effect of Zolpidem. Management: Reduce the Intermezzo brand sublingual zolpidem adult dose to 1.75 mg for men who are also receiving other CNS depressants. No such dose change is recommended for women. Avoid use with other CNS depressants at bedtime; avoid use with alcohol. Consider therapy modification
Adverse Reactions
Frequency not always defined. Frequencies noted in patients receiving other anticonvulsants:
>10%:
Central nervous system: Drowsiness (17%), dizziness (13%)
Gastrointestinal: Anorexia (13%)
1% to 10%:
Cardiovascular: Facial edema (1%)
Central nervous system: Headache (10%), agitation (9%), irritability (9%), fatigue (7% to 8%), tiredness (7%), confusion (6%), depression (6%), insomnia (6%), lack of concentration (6%), memory impairment (6%), speech disturbance (2% to 5%), ataxia (≥1% to 6%), decreased mental acuity (4%), anxiety (3%), nervousness (2%), schizophreniform disorder (2%),convulsions (≥1%), hyperesthesia (≥1%), seizure (1%), status epilepticus (1%), hypotonia (≤1%), hyperthermia
Dermatologic: Skin rash (1% to 3%), bruising (2%), pruritus (≥1%), hypohidrosis (children), Stevens-Johnson syndrome, toxic epidermal necrolysis
Endocrine & metabolic: Metabolic acidosis
Gastrointestinal: Nausea (9%), abdominal pain (6%), diarrhea (5%), dyspepsia (3%), weight loss (3%), constipation (2%), dysgeusia (2%), xerostomia (2%), vomiting (≥1%)
Hematologic & oncologic: Agranulocytosis, aplastic anemia
Neuromuscular & skeletal: Paresthesia (4%), abnormal gait (≥1%), tremor (≥1%), weakness (≥1%)
Ophthalmic: Diplopia (6%), nystagmus (4%), amblyopia (≥1%)
Otic: Tinnitus (≥1%)
Renal: Nephrolithiasis (4%, children 3% to 8%), increased blood urea nitrogen
Respiratory: Rhinitis (2%), increased cough (≥1%), pharyngitis (≥1%)
Miscellaneous: Flu-like syndrome (4%), accidental injury (≥1%)
<1%, postmarketing, and/or case reports: Abnormal dreams, acne vulgaris, albuminuria, alopecia, altered sense of smell, amenorrhea, anemia, aphthous stomatitis, apnea, arthralgia, arthritis, atrial fibrillation, bladder calculus, bladder pain, bradycardia, brain disease, cardiac failure, cerebrovascular accident, chest pain, cholangitis, cholecystitis, cholelithiasis, cholestatic jaundice, colitis, conjunctivitis, deafness, decreased libido, dehydration, diaphoresis, DRESS syndrome, duodenitis, dysarthria, dyskinesia, dysphagia, dyspnea, dystonia, dysuria, eczema, edema, esophagitis, euphoria, facial paralysis, fecal incontinence, flank pain, flatulence, gastritis, gastroenteritis, gastrointestinal ulcer, gingival hemorrhage, gingival hyperplasia, gingivitis, glaucoma, glossitis, gynecomastia, hematemesis, hematuria, hemoptysis, hirsutism, hyperkinesia, hypermenorrhea, hyperreflexia, hypersensitivity reaction, hypertension, hypertonia, hypoglycemia, hypokinesia, hyponatremia, hypotension, immunodeficiency, impotence, increased creatine phosphokinase, increased lactic dehydrogenase, increased serum alkaline phosphatase, increased serum ALT, increased serum creatinine, increased thirst, iritis, leg cramps, leukopenia, lupus erythematosus, lymphadenopathy, maculopapular rash, malaise, mastitis, melena, microcytic anemia, movement disorder, myalgia, myasthenia, myoclonus, neck stiffness, neuropathy, nocturia, oculogyric crisis, oral mucosa ulcer, palpitation, pancreatitis, peripheral edema, peripheral neuritis, petechia, photophobia, polyuria, psychomotor disturbance, pulmonary embolism, pustular rash, rectal hemorrhage, rhabdomyolysis, stomatitis, suicidal behavior, suicidal ideation, syncope, tachycardia, thrombocytopenia, thrombophlebitis, twitching, urinary frequency, urinary incontinence, urinary incontinence, urinary retention, urinary urgency, urticaria, vascular insufficiency, ventricular premature contractions, vertigo, vesiculobullous dermatitis, visual field defect, weight gain, xeroderma
Warnings/Precautions
Concerns related to adverse effects:
- CNS effects: Significant CNS effects include psychiatric symptoms (eg, depression, psychosis), psychomotor slowing (eg, difficulty with concentration, speech or language problems), and fatigue or somnolence; may occur within the first month of treatment, most commonly at doses of ≥300 mg/day. May cause sedation, which may impair physical or mental abilities; patients must be cautioned about performing tasks which require mental alertness (eg, operating machinery or driving).
- Metabolic acidosis: Use may be associated with the development of metabolic acidosis (generally dose-dependent) in certain patients; predisposing conditions/therapies include renal disease, severe respiratory disease, diarrhea, status epilepticus, ketogenic diet, and other medications. Metabolic acidosis can occur at doses as low as 25 mg daily. Serum bicarbonate should be monitored prior to initiation and during therapy; if metabolic acidosis occurs, consider decreasing the dose or tapering the dose to discontinue. If use continued despite acidosis, alkali treatment should be considered. Untreated metabolic acidosis may increase the risk of developing nephrolithiasis, nephrocalcinosis, osteomalacia, or osteoporosis.
- Multiorgan hypersensitivity reactions: Potentially serious, sometimes fatal drug reaction with eosinophilia and systemic symptoms (DRESS), also known as multiorgan hypersensitivity reactions, have been reported. Monitor for signs and symptoms (eg, fever, rash, lymphadenopathy, facial swelling, eosinophilia) in association with other organ system involvement (eg, hepatitis, nephritis, hematological abnormalities, myocarditis, myositis). Evaluate immediately if signs or symptoms are present. Discontinue therapy if alternative cause cannot be established.
- Renal effects: Creatinine and BUN elevations have been reported; monitor renal function and discontinue therapy if acute renal failure or significant sustained increase in creatinine/BUN concentration occurs. Kidney stones have also been reported.
- Suicidal ideation: Pooled analysis of trials involving various antiepileptics (regardless of indication) showed an increased risk of suicidal thoughts/behavior (incidence rate: 0.43% treated patients compared to 0.24% of patients receiving placebo); risk observed as early as 1 week after initiation and continued through duration of trials (most trials ≤24 weeks). Monitor all patients for notable changes in behavior that might indicate suicidal thoughts or depression; notify healthcare provider immediately if symptoms occur.
- Sulfonamide (“sulfa”) allergy: The FDA-approved product labeling for many medications containing a sulfonamide chemical group includes a broad contraindication in patients with a prior allergic reaction to sulfonamides. There is a potential for cross-reactivity between members of a specific class (eg, two antibiotic sulfonamides). However, concerns for cross-reactivity have previously extended to all compounds containing the sulfonamide structure (SO2NH2). An expanded understanding of allergic mechanisms indicates cross-reactivity between antibiotic sulfonamides and nonantibiotic sulfonamides may not occur or at the very least this potential is extremely low (Brackett 2004; Johnson 2005; Slatore 2004; Tornero 2004). In particular, mechanisms of cross-reaction due to antibody production (anaphylaxis) are unlikely to occur with nonantibiotic sulfonamides. T-cell-mediated (type IV) reactions (eg, maculopapular rash) are less well understood and it is not possible to completely exclude this potential based on current insights. In cases where prior reactions were severe (Stevens-Johnson syndrome/TEN), some clinicians choose to avoid exposure to these classes.
Disease-related concerns:
- Hepatic impairment: Use with caution in patients with hepatic impairment.
- Renal impairment: Do not use in patients with renal impairment (GFR <50 mL/minute).
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
- Pediatric: Decreased sweating (oligohydrosis) and hyperthermia requiring hospitalization have been reported in children; use with caution when used in combination with other drugs that may predispose patients to heat-related disorders (eg, anticholinergics). Pediatric patients may be at an increased risk for and have more severe metabolic acidosis when compared with adults. Untreated metabolic acidosis may increase the risk of developing nephrolithiasis and/or nephrocalcinosis, osteoporosis and/or osteomalacia (possibly resulting in rickets), and may decrease growth rates.
Other warnings/precautions:
- Withdrawal: Anticonvulsants should not be discontinued abruptly because of the possibility of increasing seizure frequency; therapy should be withdrawn gradually to minimize the potential of increased seizure frequency, unless safety concerns require a more rapid withdrawal.
Monitoring Parameters
Metabolic profile, specifically BUN, serum creatinine; serum bicarbonate (prior to initiation and periodically during therapy); suicidality (eg, suicidal thoughts, depression, behavioral changes); decreased sweating, elevated body temperature especially in warm or hot weather (particularly in pediatric patients)
Pregnancy
Pregnancy Considerations
Zonisamide crosses the placenta and can be detected in the newborn following delivery (Kawada 2002; Shimoyama 1999). Information related to pregnancy outcomes following maternal use of zonisamide is limited (Hernández-Díaz 2014; Kanemoto 2007; Kondo 1996; Ohtahara 2007). Metabolic acidosis is an adverse effect of zonisamide therapy; newborns exposed to zonisamide in utero should be monitored for transient metabolic acidosis after birth and pregnant women taking zonisamide should be monitored and treated as nonpregnant patients. In general, maternal polytherapy with antiepileptic drugs may increase the risk of congenital malformations; monotherapy with the lowest effective dose is recommended. Newborns of women taking antiepileptic medications may be at an increased risk of adverse events (Harden and Meader 2009).
Zonisamide clearance may increase during pregnancy, requiring dosage adjustment (Oles 2008; Reisinger 2013). Women of childbearing potential are advised to use effective contraception during therapy. Until additional data is available, other agents may be preferred for the treatment of epilepsy in pregnant women (Ohtahara 2007).
Patients exposed to zonisamide during pregnancy are encouraged to enroll themselves into the NAAED Pregnancy Registry by calling 1-888-233-2334. Additional information is available at http://www.aedpregnancyregistry.org.
Patient Education
What is this drug used for?
- It is used to treat seizures.
- It may be given to you for other reasons. Talk with the doctor.
Frequently reported side effects of this drug
- Loss of strength and energy
- Fatigue
- Nausea
- Abdominal pain
- Headache
- Lack of appetite
- Diarrhea
- Trouble sleeping
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Severe sulfonamide reaction like rash; red, swollen, blistered, or peeling skin; red or irritated eyes; sores in your mouth, throat, nose, or eyes; fever, chills, or sore throat; cough that is new or worse; loss of strength and energy; any bruising or bleeding; or liver problems like dark urine, feeling tired, not hungry, upset stomach or stomach pain, light-colored stools, throwing up, or yellow skin or eyes
- Acidosis like confusion, fast breathing, fast heartbeat, arrhythmia, severe abdominal pain, nausea, vomiting, fatigue, shortness of breath, or feeling very tired or weak
- Depression like thoughts of suicide, anxiety,, agitation, irritability, panic attacks, mood changes, behavioral changes, or confusion
- Kidney stone like back pain, abdominal pain, or blood in the urine
- Change in balance
- Lack of sweat
- Persistent or high fever
- Persistent or high fever
- Sensing things that seem real but are not
- Seizures
- Trouble focusing
- Trouble speaking
- Vision changes
- Trouble with memory
- Bone pain
- Severe muscle pain
- Severe muscle weakness
- Swollen glands
- Chest pain
- Change in amount of urine passed
- Not passing urine
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.