Dosage Forms
Excipient information presented when available (limited, particularly for generics); consult specific product labeling.
Tablet, Oral:
Otezla: 30 mg
Tablet Therapy Pack, Oral:
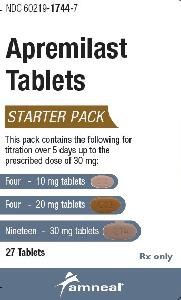
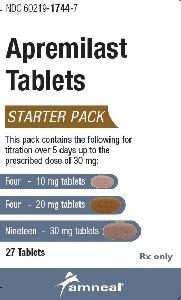
Otezla: 10 & 20 & 30 mg (55 ea)
Pharmacology
Mechanism of Action
Apremilast inhibits phosphodiesterase 4 (PDE4) specific for cyclic adenosine monophosphate (cAMP) which results in increased intracellular cAMP levels and regulation of numerous inflammatory mediators (eg, decreased expression of nitric oxide synthase, TNF-α, and interleukin [IL]-23, as well as increased IL-10) (Schafer, 2012).
Pharmacokinetics/Pharmacodynamics
Absorption
Well absorbed
Distribution
Vd: 87 L
Metabolism
Hepatic, primarily via CYP3A4: minor pathways include CYP1A2 and CYP2A6
Excretion
Urine (58%; 3% unchanged drug); feces (39%; 7% unchanged drug)
Time to Peak
~2.5 hours
Half-Life Elimination
~6 to 9 hours
Protein Binding
~68%
Use in Specific Populations
Special Populations: Renal Function Impairment
The AUC and Cmax of apremilast increased by ~88% and 42%, respectively, in patients with severe renal impairment.
Special Populations: Elderly
The apremilast exposure in elderly subjects (65 to 85 years of age) was about 13% higher in AUC and about 6% higher in Cmax than in younger subjects (18 to 55 years of age).
Special Populations: Gender
The extent of exposure in females was about 31% higher and Cmax was about 8% higher than that in male subjects.
Use: Labeled Indications
Behçet disease: Treatment of oral ulcers associated with Behçet disease.
Psoriasis: Treatment of patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
Psoriatic arthritis: Treatment of patients with active psoriatic arthritis.
Contraindications
Hypersensitivity to apremilast or any component of the formulation.
Canadian labeling: Additional contraindications (not in US labeling): Pregnancy; breastfeeding.
Dosage and Administration
Dosing: Adult
Behçet disease, psoriasis, or psoriatic arthritis: Oral: Initial: 10 mg in the morning on day 1. Titrate upward by additional 10 mg per day on days 2 to 5 as follows: Day 2: 10 mg twice daily; Day 3: 10 mg in the morning and 20 mg in the evening; Day 4: 20 mg twice daily; Day 5: 20 mg in the morning and 30 mg in the evening. Maintenance dose: 30 mg twice daily starting on day 6.
Dosing: Geriatric
Refer to adult dosing.
Administration
Oral: Administer without regard to food. Do not crush, chew, or split tablets.
Storage
Store below 30°C (86°F).
Apremilast Images
Drug Interactions
Bosentan: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Moderate): May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
CYP3A4 Inducers (Strong): May decrease the serum concentration of Apremilast. Avoid combination
Dabrafenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Seek alternatives to the CYP3A4 substrate when possible. If concomitant therapy cannot be avoided, monitor clinical effects of the substrate closely (particularly therapeutic effects). Consider therapy modification
Deferasirox: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Erdafitinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Ivosidenib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Lorlatinib: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Management: Avoid concurrent use of lorlatinib with any CYP3A4 substrates for which a minimal decrease in serum concentrations of the CYP3A4 substrate could lead to therapeutic failure and serious clinical consequences. Consider therapy modification
Riociguat: Apremilast may enhance the hypotensive effect of Riociguat. Management: Riociguat is contraindicated with nonselective phosphodiesterase (PDE) inhibitors and PDE type 5 inhibitors. Other types of PDE inhibitors are not contraindicated, but caution is advised and patients should be monitored for hypotension. Monitor therapy
Sarilumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Siltuximab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Tocilizumab: May decrease the serum concentration of CYP3A4 Substrates (High risk with Inducers). Monitor therapy
Adverse Reactions
Frequency not always defined.
Central nervous system: Tension headache (7%), headache (6%), fatigue (3%), depression (2%), insomnia (2%), migraine (2%), paresthesia (<2%)
Dermatologic: Skin rash (<2%), folliculitis (1%)
Endocrine & metabolic: Weight loss (5% to 10% of body weight: 10% to 12%; ≥10% of body weight: 2%)
Gastrointestinal: Diarrhea (18%), nausea (17%), vomiting (4%), decreased appetite (3%), dyspepsia (3%), abdominal distress (2%), abdominal pain (2%), frequent bowel movements (2%), upper abdominal pain (2%), abdominal distention (<2%), gastroesophageal reflux disease (<2%)
Hypersensitivity: Hypersensitivity reaction (<2%)
Infection: Influenza (<2%), tooth abscess (1%)
Neuromuscular & skeletal: Back pain (2%), arthralgia (<2%), muscle spasm (<2%), myalgia (<2%)
Respiratory: Upper respiratory tract infection (8%), nasopharyngitis (7%), sinusitis (2%), bronchitis (<2%), cough (<2%), pharyngitis (<2%), rhinitis (<2%), sinus headache (<2%)
<1%: Atrial fibrillation, exacerbation of psoriasis (rebound following discontinuation), severe diarrhea, suicidal ideation, tachyarrhythmia
Warnings/Precautions
Concerns related to adverse effects:
- GI effects: Severe diarrhea, nausea, and vomiting have been reported, usually observed within the first few weeks of initiating therapy. Monitor patients who are more susceptible to complications of diarrhea or vomiting; use with caution in elderly patients (≥65 years) and patients taking medications that may lead to volume depletion or hypotension. Symptom improvement observed with dose reduction or discontinuation of therapy; consider dose reduction or suspension of therapy if severe symptoms occur.
- Neuropsychiatric effects: Neuropsychiatric effects (eg, depression, suicidal ideation, mood changes) have been reported. Use with caution in patients with a history of depression and/or suicidal thoughts /behavior. Instruct patients/caregivers to report worsening psychiatric symptoms and consider risks/benefits of continuation of therapy in such patients.
- Weight loss: May cause weight loss; monitor weight regularly. Discontinuation of therapy should be considered with unexplained or significant weight loss.
Disease-related concerns:
- Renal impairment: Use with caution in renal impairment. Systemic exposure is increased in patients with severe renal impairment (CrCl <30 mL/minute); dosage reduction is recommended.
Concurrent drug therapy issues:
- Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Monitoring Parameters
Monitor weight regularly during therapy; renal function; signs or symptoms of mood changes, depression, or suicidal thoughts
Pregnancy
Pregnancy Considerations
Due to potential toxicity and limited information related to use in pregnancy, use is not currently recommended in pregnant females (Rademaker 2018).
Discontinue use ≥2 days prior to a planned pregnancy (Rademaker 2018).
A registry is available for women exposed to apremilast during pregnancy (877-311-8972).
Patient Education
What is this drug used for?
- It is used to treat psoriatic arthritis.
- It is used to treat plaque psoriasis.
- It is used to treat oral ulcers caused by Behçet disease.
Frequently reported side effects of this drug
- Headache
- Weight loss
- Abdominal pain
- Back pain
- Joint pain
- Common cold symptoms
Other side effects of this drug: Talk with your doctor right away if you have any of these signs of:
- Depression like thoughts of suicide, anxiety, emotional instability, agitation, irritability, panic attacks, mood changes, behavioral changes, or confusion
- Severe diarrhea
- Nausea
- Severe or persistent vomiting
- Signs of a significant reaction like wheezing; chest tightness; fever; itching; bad cough; blue skin color; seizures; or swelling of face, lips, tongue, or throat.
Note: This is not a comprehensive list of all side effects. Talk to your doctor if you have questions.
Consumer Information Use and Disclaimer: This information should not be used to decide whether or not to take this medicine or any other medicine. Only the healthcare provider has the knowledge and training to decide which medicines are right for a specific patient. This information does not endorse any medicine as safe, effective, or approved for treating any patient or health condition. This is only a brief summary of general information about this medicine. It does NOT include all information about the possible uses, directions, warnings, precautions, interactions, adverse effects, or risks that may apply to this medicine. This information is not specific medical advice and does not replace information you receive from the healthcare provider. You must talk with the healthcare provider for complete information about the risks and benefits of using this medicine.